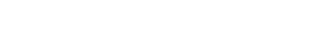Note: The authors are proud to present the findings of a study we conducted with funds from the first grant awarded from ADI’s Research Endowment, created to further the advancement of knowledge in the craft distilling industry. Our study aimed to find a method to better identify and quantify flavor congeners during the distillation of spirits.
The Problem: Which Congeners Are in Your Distillate?
Evaporating flavor compounds during distillation creates complex mixtures of volatiles that are challenging to separate and measure. Due to the expense and complexity of using advanced analytical techniques to measure flavor compounds, craft distillers rely on the time-tested method of “finger dipping and sniffing” as condensate emerges from the still. To use trade terms, the distiller seeks and individually collects the rather “rank” heads (first fraction of the spirit run), the quite “pleasantly aromatic” hearts (second fraction) and then the decidedly “skanky” tail fraction (third fraction) of a spirit run.
As a distiller of eau de vie-style brandies, I always had a lingering doubt if I was cutting too late in my heads and maybe missing some of the bright fruit essences. Furthermore, cutting the tails too early would deny my consumers a little bit of earthy ripeness in the spirit. The development of a technology to identify individual congeners and their concentrations in real time would be a panacea to the artists who dedicate themselves to distillation. Once the congeners are known, then purified congeners could be purchased and then blended to make sniffing mixtures for the purposes of training spirits judges as to where “optimum” and “subpar” exist on the aroma/flavor spectrum.
Processes for Identifying Chemical Compounds
The two standard processes for identifying compounds in a mixture are chromatography and spectroscopy. Chromatography uses various techniques to isolate chemical compounds from a mixture. The process can be readily observed and appreciated if one draws a dot of blue ink on a paper towel and then puts a drop of any alcohol (e.g., rubbing alcohol) on the ink spot. The paper acts as a chromatograph to separate the various color inks used to make blue (cyan and magenta). The alcohol solvent slowly soaks into the dry paper towel, and the various colored chemicals interact differently with the paper. They travel through the paper at different speeds and thus separate.
Spectroscopy differs from chromatography by using light-absorption signatures of various molecules to determine their identities. Current industrial-scale distillers rely on a spectroscopy technique called mass spectroscopy that measures molecular mass to identify individual compounds and their concentrations. Although mass spectroscopy is a powerful technique, it is burdened with slow turnaround (time needed to get an answer) and it can only identify compounds for which the spectroscopist has previously created a “fingerprint.” Additionally, distinguishing molecules with the same molecular formula (isomers) can be very difficult.
The purpose of our project was to examine the utility of a novel form of spectroscopy called molecular rotational resonance (MRR). Robin Felder produced the spirits at Monte Piccolo Distillery, and Brooks Pate analyzed them at the University of Virginia. MRR improves on mass spectroscopy by using pulsed microwave radiation to measure the precise three-dimensional, or rotational, structure of each molecule in a mixture. MRR can decipher the signature spectral pattern of every molecule in a volatilized mixture of compounds in real time. The unambiguous identification of complex molecules without interference from the sample matrix is unique to the MRR class of instruments based on the NIST compact Balle-Flygare spectrometer design (Fig. 1).

Two studies were each conducted over two semesters by a team of spectroscopy students in the undergraduate chemistry program at the University of Virginia. One set of experiments focused on commercially obtained peach essences in preparation for the subsequent analysis of peach brandy. The second set of experiments examined Burford pear fractions (distilled from Burford pears grown in Monte Piccolo’s own orchards). A total of 10 pear congeners were measured in the Burford pear heart fractions of an extended second distillation run of stripped pear mash.
Peach Analysis
Using mass spectrometry, a previous study of peach brandy determined that key flavor components from 18 peach cultivars and lactones in the C8–C12 size range appear to produce much of the characteristic peach flavor (Fig. 2). These lactones are present in different ratios across peach cultivars and, as a result, these ratios may help identify the ideal peach for brandy distillation. We collected rotational spectra of octa-, deca- and dodecalactone isomers to provide a library of possible flavor congeners. Our ultimate goal was to analyze blood peach distillate. Rapid monitoring of these flavor components with a cavity-enhanced Fourier-transform microwave spectrometer using a pre-programmed sample profile has been demonstrated on the BrightSpec One MRR (Fig. 1).
Pear Analysis
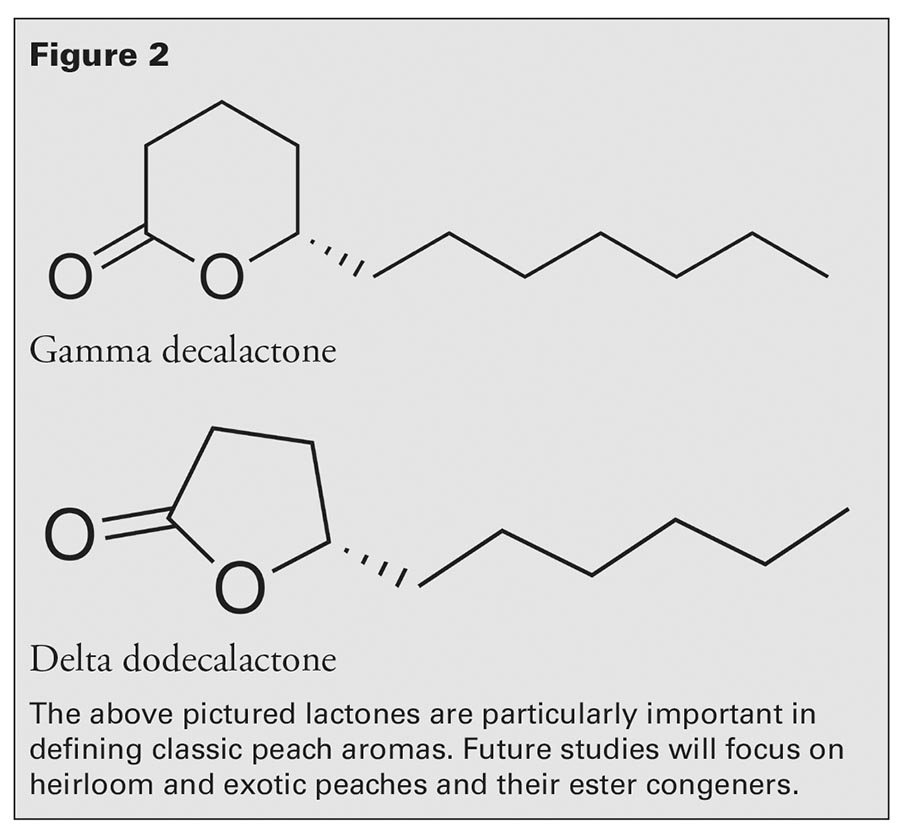
Bartlett pears (also known as Williams pears) are the principle pears used to make European eau de vie-style brandies. We knew from previously published studies that the signature aroma that characterizes Bartlett pear brandy is ethyl 2,4-decadienoate (2,4D), which is a molecule containing 10 carbon atoms and two double bonds (Fig. 3).4 If 2,4D is present in the distilled brandy at a concentration above the flavor threshold that can be detected by most humans, then the drinker can readily identify the source as Bartlett pear brandy.
By contrast, Monte Piccolo’s principle pear is the Burford. How does the Burford compare to the Bartlett? Upon analysis, we found a concentration of 2,4D below our flavor profile target. This discovery prompted Dr. Felder to plant Bartlett trees to be able to blend in Bartlett-like qualities into his brandies (i.e., a higher concentration of 2,4D). By achieving a flavor profile more similar to the European spirits that inspired him, Dr. Felder anticipates an increase in consumer demand for his brandy.
The Future of MRR in the Spirit Industry
Process management can be facilitated by using MRR either during or after spirit production. Distillers could send samples of distillate to an outside laboratory for analysis and be informed of the congeners in the spirits run as well as their temporal and time-dependent condensate elution profiles (that is, the point in the distillation where they will separate out). Future distillations with similar raw ingredients and fermentation processing would be expected to yield similar spirit quality.
Due to its unique ability to measure congener structures and concentrations in a volatile specimen, distilleries could incorporate an MRR into the vapor prior to entering the condenser. Possibilities include volatile monitoring just before the dephlegmator in a pot still. At this location there is an opportunity to use the data to regulate the temperature, distillation rate and congener separation using automated valves on the steam source. Continuous distillation apparatus might have automated sampling at each of the condensate effluent streams so that still temperatures at these points could be fine-tuned in real time to adjust the congener compositions. Vacuum stills could be sampled similar to the pot still, albeit at much lower temperatures allowed by distillation at lower pressures.
Or imagine the aid to post-production blending if you knew the amount of high quality congeners present in a brandy produced in a year when Mother Nature has been kind to the fruit. Lower quality years could be blended with high quality years to yield larger quantities of market-ready brandy. Determining the blending volume ratio could be done automatically instead of relying so much on the distiller’s personal preference or discretion at the time of blending.
Spirit judging might also become more systematic and optimized, because subjectivity is always a challenge. An examination of judge reliability during a major wine competition over a four-year period determined that only 10% of judges were able to replicate their score within one medal group (e.g., Chardonnay). Only 10% could reproducibly score a wine when determining whether a bronze or gold medal was awarded, and only half of the approximately 65 judges’ decisions from year to year were based solely on quality. Now, spectroscopy may not be able to ultimately define congeners and their concentrations that would consistently arrive at a quality determination — complex intramolecular interactions and the variability of individual judges’ olfactory biology may be too infinite to define. However, MRR could be used to classify a standard of quality that serves as a tool against which an unknown spirit could be compared, thus reducing the inherent subjectivity of judging any product.
In summary, molecular rotational resonance spectroscopy provides actionable insights into the compounds in your distillate. While craft distilling understandably values artisanship, being relatively blind as to what is going on in the still and how it will affect the quality of the product can strike fear in the heart of the distiller. Analytical tools have been responsible for major advances in the food and drink industries, usually with the goal of improving the consistency and quality of the final product. MRR technology can give you more control over when you cut and what you bottle.
References
- Stefan Grimme and Marc Steinmetz, “Effects of London dispersion correction in density functional theory on the structures of organic molecules in the gas phase,” Physical Chemistry Chemical Physics 15, no. 38 (2013): 16031–16042.
- D. Suenram, Jens Uwe Grabow, Andrei Zuban, and Igor Leonov, “A portable, pulsed-molecular-beam, Fourier-transform microwave spectrometer designed for chemical analysis,” Review of Scientific Instruments 70, no. 4 (1999): 2127–2135.
- Channing West et al., “Analysis of pear ester flavoring samples using broadband rotational spectroscopy.” International Symposium on Molecular Spectroscopy, Oral Presentation RH06 (2018).
- Bianca Willner, Michael Granvogl, and Peter Schieberle, “Characterization of the Key Aroma Compounds in Bartlett Pear Brandies by Means of the Sensomics Concept,” Journal of Agricultural and Food Chemistry 61, no. 40 (2013): 9583–9593.
- Robert T. Hodgson. “An Examination of Judge Reliability at a Major U.S. Wine Competition.” Journal of Wine Economics 3, no. 2 (2008): 105–113.
[ Acknowledgement: Chemical analysis was performed in the laboratory of Dr. Brooks Pate at The University of Virginia, Department of Chemistry, on specimens originating in Monte Piccolo Distillery, both in Charlottesville VA. ]