Part 2 of 3: A review of spirit in wood maturation,
with some speculations on rapid maturation and future potential
Abstract/Overview. How many trees died in the making of this article—let alone in allowing for the maturation of that last fine, aged spirit you made or consumed? Over the last 80 years, thousands of articles have been printed in hundreds, if not thousands, of copies, gracing the shelves of libraries and distillers’ collections on the overall topic of alcohol beverage maturation in wood. It is doubtful that anyone has had the time to consider them all with a desire to present a coherent and complete synthesis of this important scientific subject. Some authors have indeed published key reviews (and dissertations) after spending decades of their lives in researching this topic from its various angles, and they will be cited here. At least a standard file box worth of papers and about two dozen symposia volumes and other books and theses have been consulted for this highly-condensed account of spirits maturation. The aging of distilled spirits is such a vast topic and unlikely to ever be completely comprehended by any individual or group. Many of these works have not been readily available or known to the new distiller, so a base set of references here will also be of value. There are many different journals around the world with key contributory papers to be uncovered by the distiller and winemaker. It must be understood that there will always be more to discover from both older publications and current and future literature. Some of that knowledge will be illuminated here.
A big area of coverage has been in barrel-aged wine production (including sherry and brandy), as well as with Scotch. Other aged spirits have seen less published work detailing them. It is important, therefore, to consider a little about wine maturation and the chemistry involved and to understand the alcohol content differences between wines and spirits in wood and how that will impact final product quality and flavor (see part 1 of this review from the Fall 2017 Distiller). The solvent properties of alcohol-water mixtures are affected by the structure of water and ethanol clusters, and this depends upon the actual alcohol strength of the solution in the barrel, the bottle and the consumers’ glassware. It also depends upon congeners present in the wood and raw spirit.
This article (part 2 of 3) must be considered a highly subjective and severely trimmed-down synthesis of such a vast topic—perhaps forming the base for a more voluminous work with multiple contributors. Only the basic details could be presented in these few pages. With that said, hopefully the reader will use the ideas and details here as a base from which to further research existing literature for the vast wealth of information out there. Distillers will then hopefully be apprised of the subject and make better-informed decisions with respect to their own operations. It will be clear from the three parts of this article that rapid maturation is not something to be toyed with without wiser decisions and fuller comprehension by the distiller regarding what constitutes a mature spirit. As noted in part 1, a complete understanding of the sensory evaluation of distillates also bears on the understanding of both rapid- and traditionally-aged distilled spirits—this point bears repeating.
Part 1 considered the basic parameters in the evaluation of distilled spirits—with methods developed over 80 years ago to evaluate matured spirit quality and, with more modern instrumentation, still useful today.1, 2 Ethanol and water in spirits form highly complex structures manipulated by congeners present in the raw spirit or extracted from the wood. These affect the sensory properties and consumer acceptance of spirits. Part 2 now presents some of the chemistry (and essentially some of the background biochemistry and even a bit of physics) behind wood-based distilled spirits maturation.
Introduction: Points to Ponder
One of only a handful of in-depth, published non-thesis reviews on wood and maturation is that of J.R. Mosedale tucked away in the journal Forestry—hidden in the trees!3 A few simple quotes from Mosedale, along with a few choice comments and references, will best illustrate the nature of the problem.
“The difficulty in summarizing the effects of oak wood on the maturation of alcoholic beverages reflects the diverse range of disciplines and subjects that it involves, and the numerous approaches and motivations behind the studies undertaken.” Motivations depend upon whether you are a forester, a cooper, the distiller, etc. The subjects include biology, biochemistry, chemistry, engineering, forestry science, mechanics, microbiology and physics.
“It is perhaps not surprising therefore that there is often a notable lack of clarity in many of the studies that have examined the process of whisky maturation.” Disparate works in several languages—notably English, French, German, Japanese, Russian and those of a few other European countries—along with works in private collections and master’s and Ph.D. dissertations may provide more clarity, but bringing all the literature to bear upon this vast topic will prove an enormous task. Whether the science has been adequately carried out, and initial findings followed through on, is another facet to this issue. Also, while most research has been carried out on whiskeys, including bourbon, many publications cover brandies, then, with lesser numbers, rums and, recently, aged tequila.4-11
“Each study tends to focus on a single factor that may influence the maturation process, while frequently failing to control variation of other factors adequately.” More research is needed on all facets of this subject, so determining if similar research has already been done is essential. New distillers must also be taught to think of controls and all the variables at play in their own spirits experimentation. Some studies have been carried out only on model systems or have looked at only a subset of the many reactions. Fully reconstituted or completely intact systems, while taking years to study, will be necessary to finally come to grips with maturation issues. Though some studies dating back 50–80 years provide useful clues going forward, a forum to discuss the topic is called for, especially with the vast rise in the number of active distilleries.
“Both the solvent and the conditions under which the extractions from and within the wood occur, influence the resulting composition of the extractive.” Solvent considerations were presented in part 1 of this article. To mimic a process, distillers will need to know much more about the aging environment of the spirit in which they are interested in trying to replicate through rapid-maturation techniques. This may involve looking at barrel size and shape,12-13 the use of wood chips, etc.14-20 and delving into the earlier published literature where the studies covered different conditions and times for maturation.21-25
“Practical limitations frequently make a high degree of replication impossible for the types of study undertaken.” Many years are, of course, needed to study maturation with a need for extensive controls. New distillers should ensure that they are reading the early works in this area and dig into those early findings to find gems of use to them. There is no need to reinvent the cask wheel here! (Several earlier and more current works and theses are noted in the references section for this article.)
With an understanding about the issues involved in researching aspects of spirits maturation, we can delve into its subtopics and try to unravel some of the complexity and, hopefully, provide direction for future distilled-spirits production and indicate where much-needed research should be encouraged and supported.
Wood and Spirits—Coming Together
Maturation has been defined as the aging of one type of spirit, of a defined composition, in one type of wood.17, 18 That may be changing as oak barrels incorporating staves from different wood species are now under investigation. Historically, many raw spirits were generally considered harsh-tasting or “green” and undrinkable until the remarkable chemistry of dark and slow aging presents a product with a complex aroma profile, “harmony” in the mouth and a bright color to the spirit.26 According to Hornsey, approximately 200 substances can be extracted from oak and transformed by ethanol that will then participate in the formation of flavor, aroma and mouthfeel of the final spirit.26 It is likely that hundreds more components add to the nuanced complexity of the spirit. Some proponents of rapid maturation have started to rely on data showing complex volatile and nonvolatile chemical profiles to say they have produced desirable products with more rapid maturation. These profiles alone will not help define a true-to-style product.
Sensory evaluation carried out to a high level of sophistication is more likely to discern important (consumer-acceptable) differences between spirits matured for different time periods and different processes. A product can be defined as chemically perfect and taste awful. The human senses, while subject to significant variation based on physiological, environmental and mental states, are still the most sophisticated aroma/taste detection instruments and the arbiters of final quality. With that said, it will be important to understand the vast chemical soup—the distillate, machine and wood—that react and interact in ways only now beginning to be understood to produce desired mature spirits. An understanding of what takes place during maturation is likely more important than knowing the exact composition of the product.4, 26, 27 Seeing the whole wood for the trees here!
The Wooden Barrel or Cask—An Introduction
Part of understanding traditional maturation is found in the history of the cask or barrel and how it came to be the vessel of choice for carrying, storing and aging spirits. Also, understanding how different woods contribute to flavor and maturation and why certain oak species came to be the wood of choice for whiskey maturation adds to our comprehension and choices for distilled-spirits production. Different woods may be used in whole or part to add nuances to the final complexity or finesse of beverages. Several works are available dealing with this for the reader to peruse,17, 28, 29 and the distiller should consult regularly with cooperage houses to better understand their aging operations and the casks’ potential. Additional references cover the aging of spirits in different-sized barrels 12, 13, 30 and with different woods.5, 31 Independent Stave’s International Barrel Symposia Volumes, 32-34 and publications by the USDA Forest Products Laboratory 35 (2010) and Work 29, and references cited therein are also very useful guides. This article will discuss the basic chemistry of oak wood, though it is assumed most distillers, at least in the USA, will be using American oak as their main maturation wood; the porosity of oak wood permits evaporation and oxygenation of liquid contents contained within the casks or barrels—important for maturation reactions to occur! See below for much more on all of this.
Wood—The Chemical Composition
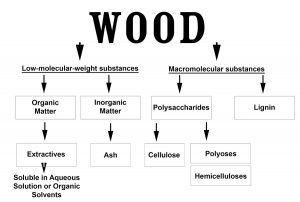
A brief account of the complex chemical composition of wood precedes a seeking out of the details of the chemistry behind maturation.26, 27, 35, 36 It should also be noted that prior contents (sherry, wine or bourbon), for those spirit types allowed to be aged in prior-use casks, will also add to the complexity of flavor and aroma. The main constituents in wood are summarized in Figure 1 and Figure 2.
Figure 1 shows the main macromolecular cell components—cellulose, polyoses (the hemicelluloses) and lignin—and introduces the general classes of low-molecular-weight materials, including extractives and minerals. The actual composition and the amounts of these latter materials will vary with wood type.36 Figure 2 then shows the so-called accessory or extractive materials in greater detail. Next, the details in Figure 3 show the major wood constituents and resultant products of their degradation. Three major macromolecular structural biopolymers of wood—cellulose, polyoses (hemicelluloses) and the lignins—and a fourth class of complex higher molecular weight polymer (the tannins) are worth considering.
Cellulose. Cellulose is the major wood component, constituting about half of both soft- and hardwoods. Cellulose is a linear and very high-molecular-weight polymer made up of glucose molecules. It functions as the main structural component of plant cell walls and keeps the cask intact. As seen in Figure 3, cellulose is not considered that important in normal maturation chemistry. One interesting note, though, is that cellulose chains can be partially degraded during seasoning and toasting to yield the disaccharide cellobiose (a sugar with two units of glucose). This sugar provides Brettanomyces wild yeasts with nutrients, which allows them to grow, resulting in spoilage of some products such as wine matured in wood.32 More details regarding cellulose may be found elsewhere for those interested.26, 36, 37
Polyoses (Hemicelluloses). Of greater importance to our understanding of maturation are the polyoses, known more commonly as the hemicelluloses.38, 39 These sugar-containing polymers are found in close association with cellulose in the plant cell wall. Unlike cellulose, there are many more different sugars involved in the polymer, and thus there are multiple ways to form the polymeric structures. Differing from the linear cellulose polymer, hemicelluloses also contain side-chain groups in addition to sugars and are often branched and, as such, are known as branched heteropolymers. Five common so-called neutral sugars are the hexoses (6-carbon sugars); glucose, mannose and galactose and the pentoses (5-carbon sugars) xylose and arabinose. In addition to these are some acids including uronic acids.36 We will see later how acidity affects the main reactions involved in maturation. In fact, these reactions cannot occur until sufficient acid release occurs from biomolecules such as polyoses. (The acid in question is likely acetic acid. See Figure 4.) One of the side groups in hemicellulose is acetic acid, which occurs once in every 7 to 10 sugar residues. During toasting reactions, in preparing wood for casks, acetic acid and methanol molecules (wood alcohol) are released.40
Lignins. Lignins are aromatic polymers that bind together cellulose microfibrils and hemicellulose molecules. Lignin has the most sophisticated structure among all wood compounds; the precise structure of native lignin is still unknown. Lignin macromolecules are composed of three different phenylpropanoid monomer units (called monolignols), predecessors or building blocks of lignin. These units are bound by various carbon-carbon and carbon-oxygen bonds. The reader can find out more about the intricate structure of lignin and its degradation products elsewhere.36, 41, 42 One structure is shown in Figure 5. The key to note here is that the base building blocks of lignins and various degradation products present active chemical groups, which play a huge role in oxidation-reduction reactions and/or flavor production. Numerous books and papers from many disciplines, including the health sciences, cover this topic in considerable detail; some of the molecules that build up lignin or are derived through its degradation are powerful antioxidants. As they can interact with many different chemical species, they are very important in spirit maturation.
Figure 1: General Outline of Wood-Derived Chemical Components
Molecules present in and extracted or reactive within the wood. Adapted from Fengel, D. and Wegener, G.36 Wood: Chemistry, Ultrastructure, Reactions. Kessel Verlag, Remagen.
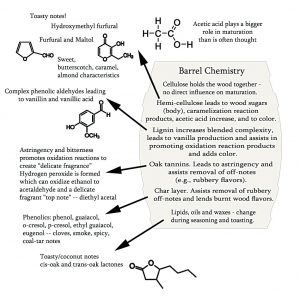
Cellulose, Hemicellulose, Lignins and Flavor. These major wood polymers in their native (intact) high-molecular-weight form do not contribute directly to aged spirit flavor. However, via hydrolytic (splitting) reactions and esterification (formation of esters), oxidation, acetylization (molecular interactions combining with acetyl radicals or acetic acid) and polymerization reactions, their structures may be modified, and these components then convey color, odor and flavor characteristics to the spirit.26
Thermochemical decomposition (pyrolysis) of cellulose and hemicellulose during coopering leads to a series of substituted heterocyclic (ring structure) compounds—furans and pyrans. the most significant being furfural and 5-hydroxymethyl furfural (HMF). (See Figures 3 and 4.) Heterocyclic compounds in relation to distilled spirits flavor have been discussed recently.43, 44
Phenolic compounds result from the degradation of lignin during the seasoning and toasting of wood and such components include gallic, syringic and vanillic acids, and the hydroxycinnamic acids, p-coumaric and ferulic acids (see below). Related compounds include hydroxybenzoic aldehydes including vanillin, syringaldehyde, coniferaldehyde and sinapaldehyde.21, 45 Other chemical species derived from wood are shown in Figures 3 and 5.
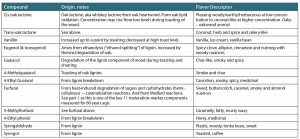
Table 1 (below) presents brief details of just some of the flavor notes derived from wood macromolecules—names, origin and flavor descriptors.41, 46-48 From sweet to caramel to spicy, herbal, coffee, phenolic and burnt, the range and subtlety of aroma and flavor from oak wood (and degree of seasoning, toasting and charring) is incredibly varied and complex. Furthermore, there are synergistic or additive effects whereby one compound can increase the perception of another or boost other aromas.47
Table 1. A few compounds, their origin and flavor descriptors. An interesting spectrum from just a few of the many wood macromolecule-derived compounds. See Figures 3, 4 and 5 and the section on Seasoning, Toasting and Charring.
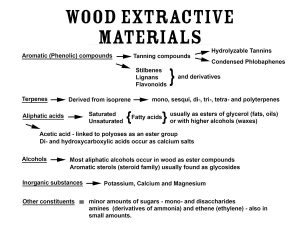
Figure 2: Accessory or Extractive Materials of Wood shows a multitude of accessory materials that can be extracted from wood. These are low-molecular-weight substances—many variant members within each class presenting hundreds of components for extraction into spirit, and which may be further manipulated via toasting and charring of the wood (see below). The contribution of chemical species to spirits, via degradation of the high-molecular-weight polymers (cellulose, lignins and hemicelluloses) and the extractive materials can be seen illustrated in Figure 3.26, 36, 40, 42, 47, 49, 50
Figure 2: Accessory or Extractive Materials of Wood
Many molecules are present and extractable from the wood. Adapted from Fengel, D. and Wegener, G.36 Wood: Chemistry, Ultrastructure, Reactions. Kessel Verlag, Remagen. The tannins and acids are more fully discussed in the text.
The Tannins. An important class of molecules derived from wood, the tannins deserve mention and are often overlooked in works on oakwood composition and in spirits-aging papers.51, 52 These are noted in Figure 2 as extractive or accessory materials, though this may belittle their importance in spirits maturation (more on this importance appears later). They are called tannins because of their ability to convert animal hides into leather. Tannins containing sufficient phenolic hydroxylic groups to form effective cross-links with proteins are considered to be good tanning agents. An extract from oak bark (Quercus spp.) is a common tanning agent. Tannins come in two broad classes; the condensed tannins or proanthocyanidins (also known more broadly as polyflavanoids and as phlobaphenes, described as reddish alcohol-soluble/water-insoluble phenolic substances) and the hydrolysable tannins. The condensed tannins are made up of building blocks called flavonoids (poly-hydroxy phenols) which are an extremely abundant and an important class of plant compounds. The hydrolysable tannins are esters of gallic acid and its dimers (digallic, ellagic acids) with monosaccharides.53
Gallotannins and ellagitannins exist and are named based upon their yield of gallic or ellagic acid upon hydrolysis. The hydrolysable tannins thus hydrolyze to yield their components; gallic and ellagic acids and sugars, usually glucose as main products.17, 54 This hydrolysis occurs readily at wine and spirit pH levels. Ellagitannins, which are described as the only hydrolysable tannin that can be extracted from oak,17 are also highly soluble in wine and spirits and contain many hydroxyl (-OH) groups. Through the hydroxy groups, these compounds are substrates for oxidative polymerization, and together with oxygen, promote accelerated oxidation and polymerization of barrel-matured wines and probably spirits.17, 55 See Figure 7 (in part 3). Ellagitannins exert a big effect upon the quality and taste of barrel-matured wine and spirits. See Figure 5 and the section: Micro-oxidation/Micro-oxygenation and Fenton Reaction Oxidation Scheme (part 3) for more on this. Other components derived from the tannins during toasting and charring processes are covered elsewhere.49-51, 56-59 Further details may be found in several works,36, 52, 53 and with a particularly useful and concise account on tannins and spirits-aging being that of Hornsey (2016). There is much more coverage of tannins, wood chemistry and maturation in part 3.26
Figure 3: Major Wood Constituents and Basic Chemistry
The different macromolecules and smaller chemical compounds from wood and the roles some of these play in spirits maturation. Figures 4 and 5 show further details of some of the chemistry involved.
Figures 1–3 illustrate most beautifully the complexity and incredible number of chemicals yielded just from the wood, especially if seasoned and treated by toasting and charring prior to spirit-filling of casks. From here we can move on to consider some wood treatments and then to the chemistry of spirit in wood maturation.
Seasoning, Toasting and Charring
Three more topics—the seasoning, toasting and charring of wood, which could form the basis of treatises of their own—can again only be touched upon here. As usual, several references will lead the reader to finding out more about how these three processes impact maturation and flavor chemistry.
With respect to seasoning of wood, natural seasoning and kilning techniques are used. In natural seasoning, wood is exposed to the elements for several years with complex biochemical changes opening up the structure and providing a set of components that can add to finesse in aged spirits. Microaerophilic fungi allow for enzymatic breakdown of the wood and such details are little publicized in the scientific literature.60-62 In effect, the wood fungi release exocellular enzymes which hydrolyze many wood components, such as ellagitannins, coumarins and polysaccharides. These facts and processes were often secret from the major distillers and the cooperage houses. It is known that, during a certain period of bourbon manufacturing, Scottish distilling experts were brought over to bourbon country to point out that the reason for poor maturation was due to the abandoning of naturally seasoned wood (personal communications between the author and industry personnel). Certainly, the distiller needs to entertain considerable discussions with cooperage experts, with some large cooperage firms offering complete classes on such topics to their clients. Oven kilning has been used to speed up the process but does not add the same level of chemical complexity to the wood.49, 50, 63-66 Charring of casks is also important, and some details may be found in the Independent Stave Company symposia volumes32-34 and elsewhere.68 Also of note, the effect of heat treatment on oakwood-extractable compounds, including lignins and tannins, and with possible application to wood chip use, was covered by Sarni, et al.69 And details on the heating of oak fragments for use in maturation can be found in Campbell, et al.70 Several suppliers are now providing toasted and charred wood products for spirits producers to bypass the cask in aging spirits. These need to be further evaluated to see how useful and reproducible they may be. For generations, French brandy producers have been making and aging wood extracts (boisé) along with their spirits for use in adding mature wood flavors to aged spirits.
Figure 4 shows the structure of a hemicellulose molecule and indicates that acetic acid residues are contained within its formula (1 molecule about every 7–10 sugar units in the chain). The backbone of a hemicellulose consists of simple sugars, about 200 per chain, and principally xylose (a five-carbon-atom monosaccharide—sugar). Upon toasting, these simple sugars are released along with some methanol. Then, under the extreme heat of toasting and under charring processes, those sugars caramelize to produce highly-flavored sweet-associated/caramelly and toasty and nutty aromatic molecules, which pass into the spirit during maturation. More details are presented in Figure 3 and elsewhere.47, 49, 67, 68
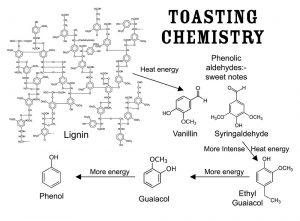
Figure 5 details some of the chemistry involved in the degradation of lignin during toasting and high-heat conditions. This is how vanillin and important phenolic aldehydes such as syringaldehyde may initially be generated under toasting conditions. Then, with more heat applied, very flavorful low-molecular-weight phenols are formed. These play major roles not only in flavor release but also in very interesting chemical reactions within the wood and aging spirit. Further details may be found in the cited literature, especially within the Independent Stave symposium books.32-34 Fengel and Wegener36 and Sarkanen and Ludwig42 provide highly detailed accounts of all classes of macromolecules found in wood. Details on the aromatic qualities of these compounds may also be found in Figure 3, Table 1, the Independent Stave books and elsewhere.41, 47
Figure 4: The Structure of a Hemicellulose Molecule and the Products Liberated During Toasting and Caramelization Processes
Sugars, acetic acid and methanol are liberated from the toasted hemicellulose molecules and then extreme heating, such as with toasting and under charring conditions, caramelizes those sugars to produce highly flavored molecules which pass into the spirit during maturation.
In concluding, the use of wood chips, spirals or staves not incorporated into barrels, or the use of different woods or barrel shapes might be entertained today.11, 14, 15, 18-20, 70, 71 These are used in place of the barrel with the aim of speeding up maturation events. The wine industry has presented some research into this area of alternate or rapid maturation, and distillers choosing this approach need to learn more about what is indeed known and to encourage further research into this topic.
Figure 5: The Partial Schematic for a Complex High-Molecular-Weight Lignin Molecule and Showing How Applied Heat Energy Leads to Lignin Degradation and Modification
Vanillin and important phenolic aldehydes such as syringaldehyde may initially be generated under toasting conditions. Then with more heat applied, very flavorful, low-molecular-weight phenols are formed. These play major roles not only in flavor release but also in very interesting chemical reactions within the wood and aging spirit. Further details may be found in the cited literature. See Table 1 for flavor details.
Concluding Part 2.
A vast complexity of wood components and a plethora of flavorful compounds derived from wood degradation are imparted to a spirit in the initial stages of maturation. In part 3, to be published in the Summer 2018 issue of Distiller, the processes and reactions inside the barrel during the maturation of spirits will be covered. The rise of rapid maturation systems, which we will see are often a resurrection of methods practiced as early as 80 years ago, and the reasons for distillers to return to traditional aging will be covered. In relation to that last point, we also return to looking at solvent chemistry, the impact of congeners on solvent structure and the influence of solvent upon congener solubility and volatility. Micro-oxygenation and oxidation chemistry, and the role of tannins, along with a proposal for a catalytic engine mechanism of spirits-aging will round out the general discussion. Finally, a summary, with conclusions, will be made, which will propose a few issues that might provide some leads in assisting in resolving the rapid-maturation dilemma.