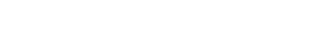Overview. This is part 3 of a treatise on distilled spirits maturation. Part 1, in the Fall 2017 Distiller, saw the coverage of ethanol-water solvent systems and key analytics for monitoring spirits maturation. Part 2, in the Winter 2017 Distiller, dealt with the difficulties of understanding maturation and then with the cask, the structure and the chemistry and flavors of oak wood constituents. Part 2 concluded with a look at seasoning, toasting and charring of oak wood. Part 3 looks at the chemistry of maturation, rapid-maturation systems, a return to tradition, solvent and congener chemistry and important oxidation reactions. It concludes with proposed mechanisms for maturation and some speculations on rapid or alternate aging systems. It will hopefully become clear that we really have probed only the tiniest scratch in the surface of this vast topic.
Processes and reactions in the wood during maturation of spirits
The process of maturation is characterized by changes in the color and flavor of the spirit with a concomitant decline in spirit volume and alcohol content. As noted in part 1 and below, the composition of the solvent dictates further maturation reactions and the perception of aromatics. There are primary aroma/flavor compounds present in the spirit (from raw materials) that carry through the entire process, secondary compounds (such as esters and fusel alcohols), which arose during fermentation and tertiary notes (phenols, lactones and much more) arising from transformations in the wood or extracted from oak. The time required for satisfactory maturation also varies according to the characteristics of the raw distillate and the size, wood origins and treatments of the cask along with the environment where the spirit is matured.1 Mosedale and Puech list the causes of the changes in flavor of maturing spirits which are due to changes in the composition and concentration of many compounds found within the system.1
- Direct extraction of wood compounds (natural lower molecular weight components, including those generated during fungal enzyme attack on wood during seasoning and upon toasting and charring of the wood)
- Decomposition of wood macromolecules (lignin, cellulose, hemicelluloses and tannins, etc.) and extraction of their products into the distillate
- Reactions between wood components and the constituents of the raw distillate
- Reactions involving only wood extractives
- Reactions involving only the distillate components
- Evaporation of volatile compounds (low boiling point compounds through the wood of the cask)
To the above we may add the need to understand flavor compound interactions—synergistic and antagonistic effects. A quick outline of the main events and activities taking place in the wood during maturation can be summarized thus:
Volatiles are lost
There are evaporative losses of water, ethanol and other volatiles from the wood. This depends upon environmental variables, humidity and temperature, leaky barrels and differential pressure: The top of the warehouse having completely different conditions from the bottom, the sunny side vs. the shady side of the warehouse, etc., also with different environmental physical conditions. Enter the art of the distiller in blending the same batch-produced spirit from casks of different final, though nuanced, spirit composition, aromas and flavors. Rapid-maturists are likely limited to the production of only much smaller batches of product each time, though may be able to reproduce their own formulations much more consistently and rapidly, making up for small-run production lots. How the final flavor matches expectations, though, is the issue.
New compounds are formed or released
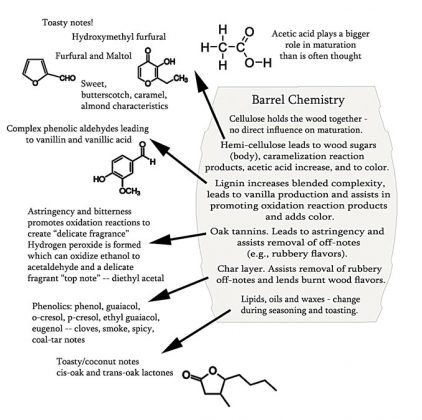
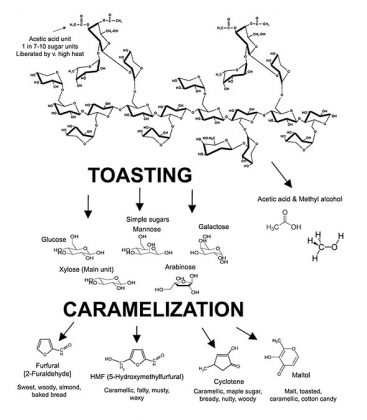
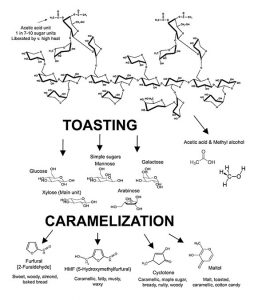
There are complex interactions of spirit and wood. These chemical interactions occur within the wood and within the spirit leading to the release of, or the generation of, many new compounds. These new compounds then participate in known chemistries and in even further, as yet unfathomable, reactions. As spirit enters the wall of the cask and returns to the bulk liquid, it carries smaller compounds back with it. Variables here include the use of new or used barrels, toast levels, char levels, how the wood was seasoned as well as the storage conditions during maturation as noted above. Carbohydrates are liberated by alcohol as monosaccharides or larger units from the wall of the cask2 (see Figure 3). The content of simple wood-derived sugars is higher in bourbon than for the Scotch and Irish whiskeys based on new cask use. Acids form an interesting and important group of volatile and non-volatile aroma compounds liberated by alcohol from oak. Monocarboxylic acids (acid functional group = COOH)—acetic acid, for example—is released from hemicellulose (see Figure 4). As noted elsewhere in the text, acetic acid can be reduced to ethanol. Dicarboxylic acids (two COOH acid groups in the molecule) add to the acidity of the maturing distillate and are important in the formation of other aroma compounds. Such acids catalyze reactions that produce flavorful esters, acetals and lactones during maturation. The dicarboxylic acids may also esterify with ethyl alcohol to form a wider variety of flavorful esters. Without the presence of many of the components derived from wood, the aging of distillates in non-wood vessels is next to impossible and it is doubtful that the full complexity of aging can be achieved or reproduced in such vessels containing only wood chips, staves, spirals or other forms of artificial means of adding such desired constituents.3-6 Yet some advances and experimental data will be discussed further below on this topic. The issues of recharging of casks may also bear investigating here—in relation to consistency of matured spirits production.7
Higher alcohols (the fusel oils) remain relatively constant throughout (traditional) maturation. The way to control their concentrations is through distillation—reducing their levels in the raw white spirit. While the higher alcohols can participate in esterification reactions, with the various acids present in the barrel, this does not seem to have a significant impact in diminishing the actual free higher alcohol levels. Other than ethyl acetate, the other esters are present in very small amounts (though are flavorful due to having a low threshold of detection levels). It is suspected that the acids and the alcohols involved in ester formation can swap and exchange with each other and this will be dictated by the principles of chemical equilibria. Whether it has a major impact on the overall maturation is probably unclear, although such reactions will be highly dependent on the overall system conditions. The kinetics of formation and reaction dynamics here are under current investigation.8, 9
Sulfur compounds
Many potently flavored compounds are lost or markedly decrease during wood aging. Foul-odor sulfur compounds are an issue for spirits producers. These losses and decreases require other events to have occurred and might not be overcome without the time factor involved. It is known, for example, that the acidity level in the barrel needs to attain a certain level before any other maturation chemistry can occur. The rise in acidity is described elsewhere in this article and may be important based on the pH-dependency of chemical reactions. Furthermore, micro-oxygenation and oxidation reactions (discussed further below) may also be important here.4, 10, 11
Degradation reactions in the wood
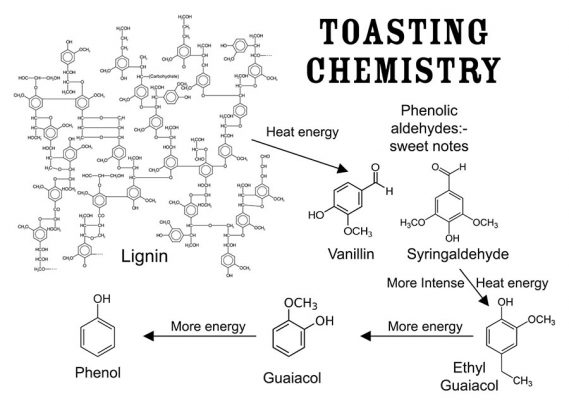
Quite a lot of degradation reactions take place in the wall of the cask during maturation.12 Both alcohol and water pass through the wall of the cask, penetrate wood cells and initiate the hydrolysis (splitting of molecules by means of water activity) of the hemicelluloses and decomposition of the macromolecular lignin compounds discussed earlier. (See Figures 4 and 5). This releases sugars, acids—especially acetic acid—and polyphenols which participate in the multitude of subsequent maturation steps in the barrel.
From the above brief comments, it will be apparent that mimicking all these processes and steps outside a barrel is thus a huge undertaking. An incredible amount of biochemistry, chemistry and physics is involved in maturation of distilled spirits. Further information on the above points may be found elsewhere.1, 3, 9, 13-17
Speeding things up by sidestepping a centuries-old formula: alcohol + oak + time = deliciousness.
A Rube Goldberg machine is a deliberately complex contraption in which a series of devices that perform simple tasks are linked together to produce a domino effect in which activating one device triggers the next device in the sequence. A similar scenario describes many biological systems and likely the situation in all the biochemical and chemical systems involved in spirits production and maturation. Reactions replace devices in the definition and follow a course of sequential chemical activities and manipulations in a very defined thermodynamic order and according to defined kinetic mechanisms. Over the years, the expression has expanded to mean any confusing or complicated system, which seems apt here. Multiple attempts have been made and many fancy systems have been applied by distillers to try to beat Mother Nature and Father Time in producing “matured” spirits in “rapid time.” Some of these have been described in the popular press, and several spirits have stood alongside traditionally aged counterparts in competitions and have earned medals of excellence and “prestigious” awards, or at least news coverage. Thirty-four such popular press articles are cited in the references section.18-51 Patents have also been issued for rapid maturation of spirits.20, 22, 40, 41, 45 Based on the complexity of maturation and lack of training in sensory evaluation, especially for understanding distilled beverage flavor, it is unlikely that these rapid-maturists have indeed reached perfection in their endeavors. If the attempt, via the use of modern processing technologies, is to produce a tasty product acceptable to the consumer, with many of the required attributes present in a reasonable harmony, then there is nothing wrong with that approach. To say they have reproduced a fully authentic and identical-tasting product to one matured over an extended aging period is a completely different story.
In a 2016 National Geographic article, Heather Brady said, “As pioneers in the world of whiskey distilling take advantage of the brown liquor’s resurgence and try to speed up the traditional oak-barrel aging process, they face a big roadblock: an old guard of distillers who refuses to share their secrets.”51 Well, the secrets might be out there in the vast literature, though the main secret may lie in the fact that the big boys figured out that rapid maturation just might not work!
Slowing things down—returning to tradition: a natural and true Rube Goldberg machine
What many craft distillers do not appreciate is that rapid-maturation experiments were carried out by the major distilling houses over 80 years ago, hinted at in Liebmann and Rosenblatt;52 Liebmann and Scherl;53 and in a few early and newer patent applications.20, 22, 40, 41, 45 Some earlier works might be considered quackery today though they also included many of the so-called newer methods developed by craft distillers.48 News flash: A lot of the so-called newer methods have been tried before!27, 48 In the interests of modern economies, if products can be brought to market faster, they will be.
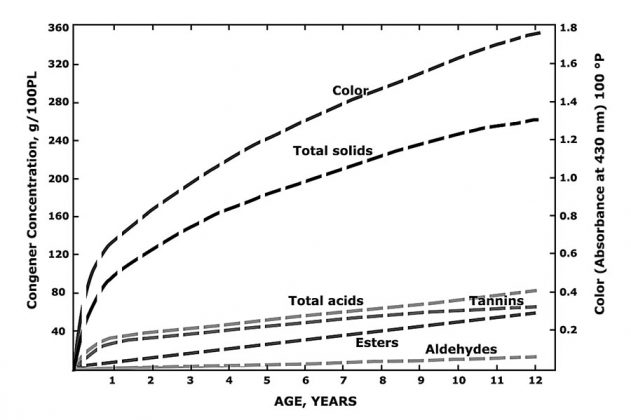
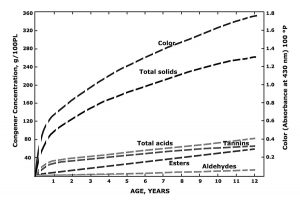
Getting back to traditional aging schemes, we consider key congener and flavor development and focus on parameters deemed important for more than 80 years of following spirits maturation. Congener development in spirits has been extensively researched since the early days, and the general mechanics of production and manipulation of a core set of the major flavor notes during aging (those with low flavor-detection threshold, which consequently have big impact on flavor) are well-understood.13,54,55 Details of such published studies, which included investigations with different barrel-strength spirits and aging periods in years, are worthy of searching out and reading.1, 13-15, 52-54, 56-58 Otherwise the modern distiller may be repeatedly reinventing the wheel. The paper by Baldwin and Andreasen54 provided a summary figure as to the development of the major congeners over the 12-year aging period they studied. This was for bourbon and a simple rendition of their figure is presented here as Figure 6. The figure shows details regarding the rate and extent of formation of the various classes of congeners and color formation.
During barrel-aging, the predominant transformation in the distillate in quantitative terms is the linear increase in ethyl acetate due to the oxidation of ethanol (this may be used as a marker for aging). Over the lengthy course of barrel-aging, ethyl acetate may increase from three to six times the amount originally present in the distillate, according to Reazin.12 In conventional barrel-aging, the pH takes about six months to drop to the required acidic levels for the various reactions necessary for the development of beverage character to occur. Thereafter, the production of ethyl acetate is dependent upon the formation of acetic acid in the barrel, which takes 36 to 48 months to reach the level of marketable, aged beverages, often 4 years old.12, 59
One patent process suggests that to accelerate the maturation process, the addition of ethyl acetate should be made.45 This would likely affect the delicate equilibria for all other species in solution and lead to a radically different flavor profile to that obtained via the natural formation and consumption of the acids, esters and alcohols in solution. Distillers should not forget that reactions are not separate events. The product of one reaction is often the substrate for another reaction and so on ad infinitum. Hundreds of compounds with differential solubilities and reactivities are present in the barrel-aging spirit. Altering one chemical component’s concentration has multiple ramifications within the chemical system. As the acidity level is important to further maturation, one must wonder why these patent-application authors did not consider altering the acidity rather than adding, (presumably chemically synthesized) ethyl acetate to the system.45
Though the points above apply to shifts caused by perturbing the delicate chemical ecosystem, such might not be the case for aging over or on wood chips, etc. In that case, adjusting the acidity of the spirit might be beneficial. We really do not know yet. This idea should be subjected to further research to see what happens. Distillers must understand the complexity of systems before adopting ideas simply pulled out of the patent-application hat or without following the details of experiments presented in the scientific literature.
An adaptation of a profile showing the development of congeners, color and solids during whiskey maturation. This schematic has appeared in more recent reviews but was originally presented by Baldwin and Andreasen.54 Esters are largely represented by ethyl acetate. Congener content is expressed in grams per 100-proof liters. See also Reazin59 for summary details.
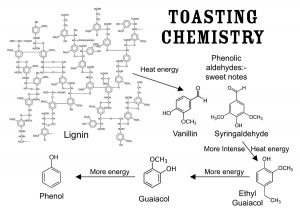
To further understanding flavor, beyond the better-known esters (fruity, solventy notes), the acids/volatile fatty acids (sour to rancid, cheesy characteristics), the lactones (whiskey lactone derived from oak, coconut and green flavors) and the higher alcohols (with harsher-solventy, bitter, nutty and floral notes) there are many other classes of compounds to be considered. These include heterocyclic compounds with green, bitter, astringent, roasted, burnt, nutty and even fishy notes.66-62 Also, phenols and phenolic compounds, including the phenolic aldehydes, vanillin, syringaldehyde, coniferaldehyde and sinapaldehyde, derived from lignin degradation products in oak barrels during coopering, should be understood. See Figure 5. These aldehydes contribute potently to the flavor of barrel-aged alcohol beverages and can be used for the identification of spirits and as aging markers based on raw material and aging times in casks.63 Many other compounds are discussed in a plethora of scientific papers, and all this detail could not be presented in such a compact review. Different spirit types—whiskeys, brandies, rums, etc.—also show their own unique sensory properties, chemical identities and unique chemical complexities. The incredible complexity of maturation has many details to note in helping distillers understand their own processes and products.
The reasons for heading back to traditional aging schemes by the major distillers over 80 years ago was likely due to the very poorly understood physics and chemistry of maturation at that time. We still only know a fraction more today! An earlier study paper worth seeking out is that of Boruff and Rittschof56—where they discussed the effects of barreling proof (110-, 118-, 127- and 154-proof) and American whiskey aging. This research alone provided a wealth of information to the bourbon industry. The summary here just scratches the surface in respect to our understanding of this complex topic. Realizing that the entire log beneath and beyond contains what we yet do not comprehend about spirit in wood maturation is quite sobering.
Interactions of alcohol and congeners and impact on aroma compounds and assessment in spirits
Part 1 of this article covered solvent properties of distilled spirits, including vodka, which begs the question, “What has vodka got to do with aging?” Well, as the bulk matrix of any spirit is made up of water and ethanol, a pure vodka vs. a white whiskey with much higher levels and types of congeners present should provide us with an excellent “control spirit” to put into the wood. A discussion of clusters or aggregations of ethanol and water was covered. Several components and their oxidation products as extracted from the wood cask may play a significant role in the stabilization of such clusters.64 Losses of ethanol and water through the cask wood65 increases the concentrations of many volatile spirit components during maturation, which impacts the solvent systems and vice versa.54, 56 Differential losses in water vs. ethanol leads to a change in proof 52 and changes in the bulk solution properties, and so the cycles continue.
The solvent properties of ethanol and ethanol-water mixtures have received extensive research treatment: During the aging studies at different proof strengths and times of aging, and during natural maturation in warehouses, considerable losses of alcohol and water take place. As the alcohol strength increases, the extraction and the reactions of aging will change. Chemical equilibria will change to effect different extents of reaction in solution and at the liquid-wood interface. If concentrations don’t change with respect to ethanol and water in modern aging systems in the same manner as they do in barrel-aging systems, one cannot expect the same extraction or solubilization of chemical species in the spirit! Years more research will be needed to better understand the impact of solvent on congeners and vice versa.
The Scottish spirits industry members were the first to define sensory evaluation of whiskeys by nosing (and tasting) as requiring dilution of spirit to 23% ABV to reduce the pungency of the alcohol and to allow the congener flavor notes to shine through. If structure and “smoothness” rely on the concentration of the alcohol, then diluting it breaks the structure and, as such, the theory cannot be tested in side-by-side comparisons of high-alcohol spirits. This is partly why defining sensory evaluation systems will be so important to judging the effectiveness of rapid maturation via assessment of final product aroma and flavor quality. And, moreover, to competition judging and consumer taste panel studies.
The perceived aroma quality of a spirit sample via nosing is of course related to the volatile aroma chemicals it contains and how they partition into the gas phase—the headspace of the bottle or glass.66 This gets into physics and physicochemical properties, and we will defer to the original publications for those interested in learning much more about all this. See the cited references of the Conner, Paterson and Piggott group, as they did the pioneering work in this area in the 1980s and 1990s.67-75 A few important points, with other references noted, include the following statements.
Medium- and long-chain ethyl esters were found to have a limited solubility when model spirits and malt distillates were diluted for sensory analysis. Conner, Paterson and Paggott said, “At concentrations above an observed critical level the concentration of esters in solution remained constant, with the excess esters then forming agglomerates.”70 Furthermore, wood extractives were seen to cause changes in the solubility of organic compounds thus affecting this system. Increased concentrations of organic molecules in agglomerates results in respectively lower solution concentrations. In consequence, reductions in free solution concentration then result in lower headspace concentration, and this influences the flavor of the matured spirit.69
A proposal in one rapid-maturation patent called for the addition of purified ethyl acetate to the spirit to mimic more traditional wood and time maturation. Remy, Delmore and Buske said, “In a maturation process for producing an aged ethanolic beverage, the improvement which comprises adding ethyl acetate prior to or during the maturation process in a quantity sufficient to accelerate the maturation.”45 Altering chemical systems is not easy; the typical amounts of compounds that are proposed to be added must be known and it needs to be understood that this will totally disrupt the status quo of the system. The point being that this type of approach will have potentially profound effects on the solubility and volatility of many other flavor-active species, as noted in the paragraph above. This will likely lead to a spirit showing considerable difference to one not having artificial modification.
For those interested in pursuing the idea of the Remy, Delmore and Buske patent45, the paper by Boothroyd, Linforth and Cook66 will be of interest. Ethyl esters or compounds of similar polarity have been shown to be correlated with immature odors in fractions of a brandy,75 which begs the question, Will this patent process then produce a product of immature flavor perception rather than a rounded fully-matured flavor profile? What is also proposed here is that as spirit ABV is decreased, ethyl esters present at higher concentrations are more likely to form agglomerates. These structures can then incorporate or “trap” small hydrophobic flavor compounds, lowering their headspace concentrations, and change the balance of the nosed aroma to more polar, water-loving compounds and the overall aroma balance9, 66 and result in a change in the “sensory characteristics of distilled beverages matured in oak casks.”75 Thus, these volatility and solubility factors impact sensory determination of the spirit. An interesting account of this was presented by Overbosch, Afterof and Haring76 and the reader is encouraged to see the works of Gonzales-Robles and Cook, Jack77 and Macatelli, Paterson and Piggott78 to better understand the complexities of sensory evaluation of spirits. Last but by no means the end of research here, in covering the dilution of whiskey from its molecular perspective and bringing us up to the minute, Karlsson and Friedman79 state: “Our findings may apply to other flavour-giving amphipathic molecules and could contribute to optimizing the production of spirits for desired tastes.” That will be a profound statement for those experimenting in rapid-maturation processes.
Micro-oxidation/Micro-oxygenation and Fenton reaction radical oxidation scheme
Most winemakers and distillers understand that something called micro-oxidation or micro-oxygenation is important to maturation of liquids in wood, though many of the details may also apply to spirits through the presence of phenolic compounds derived from the wood.10 Yet very few distillers understand much about it. In fact, the correct term should be micro-oxygenation, but because oxidation appears in much literature, oxidation and micro-oxygenation must first be defined to clear up the confusion.
Oxidation is a chemical reaction promoted by oxygen, oxygen-related species or transition metal ions. This we will cover further below. Micro-oxygenation (MOX) on the other hand, extensively studied in wine-maturation experiments and studies, is the deliberate and carefully delivered introduction of set amounts of oxygen to affect oxidation reactions in the wine or spirit.80 The MOX technique involves a controlled flow of gaseous oxygen into the solution in the form of microbubbles injected through a micro-diffuser.10 At a recent whiskey summit meeting, the use of air bubbled into a rapid-aging system was detailed, presumably to speed up important oxidation events.
Tiny amounts of oxygen play a vital role in barrel aging with impacts upon flavor, aroma and phenolic composition of aging spirts. In barrel aging, the oxygen diffuses into the wood from the ambient air surrounding the casks. For rapid maturation systems it is possible that micro-oxygenation could be used as an alternative to barrel aging when used in combination with staves, chips or exogenously added tannins. Some such rapid-maturation engine systems seem to be undergoing trials. (Much has been written on the topic, including how and when to implement MOX and applications in accelerating wine and spirits aging.10, 11, 80-85 To apply to non-barrel spirits aging, pay attention to two articles dealing with this topic; Pons, et al.86 and Waters.87 A detailed chemical account covering the phenolic compounds at play here and the influence of pH is covered in the article by Kontoudakis, et al.10).
The first step in these micro-oxygenation, and thus oxidation, schemes involves the formation of acetaldehyde (ethanal) from ethanol. Following this, a series of cascade reactions can occur through reactives known as free radicals. Details for wine aging in wood are progressing but very little is known from the literature about the overall importance of oxidation in alternate-method spirits aging.
The Fenton Reaction
Chemists and some distillers know the name the Fenton reaction and understand that it is important in spirits production. But what exactly does it do? And how does it fit with micro-oxygenation? This reaction is a metal ion-catalyzed chemical reduction of hydrogen peroxide (H2O2) which produces highly reactive chemical species known as hydroxyl radicals, which are capable of oxidizing ethanol to acetaldehyde.85, 88 Moreover, the Fenton reaction is now thought to be a key step in non-enzymatic wine and probably spirits oxidation. The reaction and its reach are not yet completely understood. Radicals are highly reactive chemicals capable of creating negative flavors from oxidation of food constituents and during aging of many wines and spirits in the wood.88 The reader should be aware that molecular oxygen itself is not very reactive so bubbling oxygen into a raw white spirit without all the required ancillary components will not work to generate required flavor compounds. The system itself, with certain chemicals derived from wood, acts to control the oxidation so that oxidative spoilage is avoided—thus the actual wood-aging system will be important in this regard.80, 85 During barrel aging, the humidity of the wood and the thickness of the grain of staves play a role in the uptake of oxygen. There will be much to control in systems using staves, chips or alternate wood products for spirits maturation—and slow oxidation seems to be the order of the day here!
Much research has again been carried out with respect to wine model systems, and the yield of acetaldehyde produced via the Fenton reaction has been shown to be remarkably reduced if oxygen is also present! This leads us to two thoughts. The control of the level of oxygen in the barrel or alternate “wood engine” needs to be better understood; micro-oxygenation being important in maturation systems. Many red wines and possibly certain distilled spirits may benefit from a limited degree of oxidation which reduces astringency and enhances color. Additionally, metal ion concentrations in the spirit and the barrel need to be better evaluated for their oxidative potential. To date, even with wine and barrel aging, research is lacking, which is unfortunate in that the quality of wines and spirits is significantly impacted by oxidation. Finally, for this section it is also noted that polyphenols, abundant in wood and leached into spirits, are also apparently required for non-enzymatic oxidation even in the presence of trace metal catalysts.88 Polyphenols are also known to complex with metal ions, which could also reduce their effective concentration and catalytic abilities. Again, the systems proposed are by no means exclusive or simple. A model idea for barrel-aging systems is presented below.
A question to be asked, leading from a comment above, should be: Where does the dangerous hydrogen peroxide come from which leads to radical production? Polyphenols are primary substrates for oxygen in wines and spirits and are major targets of so-called hydroperoxyl radicals, which arise from reactions between oxygen and iron and/or copper ions. The radical attack leads to hydrogen peroxide production which then likely reacts with iron or copper ions in the Fenton reaction to produce the hydroxyl radicals. This species is capable of nonspecifically oxidizing virtually all organic components in proportion to their abundance. Certain other chemicals such as sulfur dioxide in wines can exert a protective effect against such further oxidative damage. The point here is that we do not yet know enough about these reactions in wood to know what occurs during either maturation processes—traditional or rapid. Ellagitannins are important oxidation regulators and facilitate hydroperoxidation of wine (and spirit?) constituents. The chemical details for wines, and the role of ellagitannins and gallic acid in Fenton-type systems and control of the oxidation-reduction potential in aging wines and spirits can be reviewed elsewhere.80, 89, 90-92
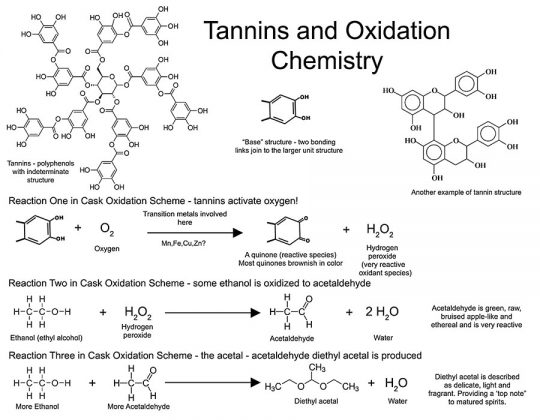
Figure 7 shows two examples of the structure of tannins and oxidation reactions carried out in their presence. Note the structure on the right. We may recall from part 2 that tannins form another complex chemical group, and are largely constructed by condensation of polyhydroxy phenols (flavonoid class) or phenolic acids (e.g., gallic acid) and show widely different properties based upon how they are constructed (see The Tannins section in part 2 and Hemingway and Karchesy93). Tannins can activate oxygen in the presence of certain metal ions to generate energetically active compounds—namely quinones (oxidized derivatives of aromatic compounds) and a very reactive pro-oxidant, hydrogen peroxide (H2O2)! The peroxide can then oxidize ethanol to acetaldehyde (also a reactive species). Ethanol and acetaldehyde can then react to form an acetal—diethyl acetal, which may convey a delicate fragrance (a “top note” to spirits)—lending a higher quality and a more pleasing spirit. As metal ions are involved in catalytically small doses, any nontraditional system that does not supply these ions may not produce such refined notes to a spirit. Also, diethyl acetate is only one of many interesting species generated through such oxidation reactions—the Fenton reaction “creates an inventory of aldehydes and ketones.”85 See also du Toit.80 So much for wine chemistry; an understanding of the very complex Fenton reaction in spirits maturation has seen little in the way of published data. Clearly though, wood extractives of a certain variety—the ellagitannins, at the very least—would be important in barrel-free aging systems, and studies should be directed in this area to better comprehend the possibilities of full spirits maturation in nontraditional aging schemes.
A Catalytic Engine?
The charring of wood leads to many crevices, increasing the overall active surface area of the barrel. Toasting and the earlier seasoning of the wood will yield many chemically active species which may present their chemical groups, especially perhaps the hydroxyl groups of the tannins, polyphenols, etc., within these micro-sites or crevices. At a very simplistic level, this is the situation with enzyme chemistry providing active chemical components that can react with temporarily harnessed, or trapped, substrates. Non-enzyme catalysts work in the same way by providing surface active groups for localized concentration of components for chemistry to occur. Biological catalysts—the enzymes are also enhanced in their abilities by carefully placed metal ions and acid-base chemistry—are key players in such catalytic chemistry. Bring in a solvent (the distilled spirit) and with all suitable components present (including oxygen, a suitable pH and organic acids, etc.), it must surely be possible to consider the barrel as a massive, chemical catalytic engine—subject to thermodynamic principles and chemical kinetics. Early life chemistry is said to have started by localized concentration of suitable molecules on the surface of primitive clays and through interaction of surface-exposed chemical groups. This is appealing as an explanation to the recharging of exhausted barrels. Exhausted barrels are likely reduced in catalytic potential strength as a result of leaching of components into the spirit, reduced size of crevices as they are washed away, or when certain sequential reactions have completed their life cycles and established chemical equilibrium or substrates are finally depleted. Re-charring barrels may open up a fresh layer of appropriate chemical groupings and re-create crevices for further activity to occur when recharged with fresh new make spirit and acidity has reached a desirable level (Putman covers details as to reactivating casks that may bear upon this idea7).
For a reaction to occur in solution, the reactants must encounter one another by random collision and thus by chance encounters. Marginal increases in the rate of such collisional encounters may occur upon raising the temperature, and this may be the case during thermal changes in the warehousing of spirit in barrels or by application of heat. Such conditions could be applied in non-barrel systems. However, when substrates are sequestered within the active site of an enzyme, or perhaps in “pockets/crevices” in a catalyst or charred wooden barrels, the effective concentration of reactants is greatly increased. If the reactants are also placed in very close contact and in the optimal orientation, that also greatly speeds up a reaction. Of course, the products must diffuse away rapidly, otherwise the catalytic center gets blocked or saturated and subsequent reactions at that site cannot occur readily. Notable here is that spirit does diffuse in and out of the barrel wood surface layers! Thus, saturation or flooding will likely not occur at the barrel surface; equilibrium will not then readily occur due to product-substrate depletion. This proximity effect is certainly an interesting idea here for the complex system of the spirit-in-wood maturation engine.94-96
Hydrogen bonding, electrostatic and hydrophobic interactions in the wood crevices can distort substrates, pulling them closer to the transition state needed for the actual reaction to occur.94 Many of the wood-derived molecules, especially the phenolics, carry the appropriate chemical groups for simple catalytic events to occur and, as oxygen diffuses into the wood, maybe the tannin-based oxidation reactions we see illustrated in Figure 7 could take place. Price and Stevens96 discuss chemical species which react that are not unlike those found in the spirit-wood system, including lactone formation which is an internal esterification, so again these seem to be not unreasonable propositions. As an aside, it is noted that whiskey lactones play an important role in the flavor of many oak-aged spirits. Finally, many reactions of the type catalyzed by enzymes are also known to be catalyzed by acids and/or bases, including acidic metal ions (note the role of metal ions in Figure 7 and see also Ramey and Ough,97 who discuss minor effects of acid catalysis upon volatile ester hydrolysis and cite several other key chemical kinetic works in the area). Price and Stevens96 show examples that could apply to the chemical reactions in the wood, and we have already noted that until the acidity level reaches a certain level in the barrel-aging spirit, supposedly none of the other maturation reactions can occur.
The questions become: How much combinatorial and reaction chemistry is involved in the interface concentration of spirit components with the barrel or wood fragments, chips, etc.? Does charring of the wood surface create “catalytic centers” with exposed hydroxyl groups (e.g., from polyphenolics or any other of the myriad of complex biomolecules present in the wood) which could participate in oxidation and acid-base catalytic chemistries? Is this how recharging exhausted barrels works? Metal ions also interact with polyhydroxyphenols and could also promote catalysis through such combinations of active sites, reactive chemical groups, acidity, oxygen tension, etc. Will alternate systems ever mimic the barrel in this respect? We leave this intriguing topic alone at this point, but see Copeland;94 Cornish-Bowden95 and Price and Stevens96 for much more on this fascinating topic.
Conclusion
This article in three parts has now provided a comprehensive, though hopefully succinct and clear, account of some of the variables and principles involved in the maturation of distilled spirits. The discussion also includes some controversial and untested ideas that maybe are worthy of further investigation.
An education and discovery of the details published in the extensive literature will certainly not harm the seeker of rapid-aging nirvana. Though, as noted within, we barely even understand traditional maturation, despite decades of studies and a multitude of published papers on the topic. The rates of formation (the kinetics) of compounds during aging require further investigation, especially for alternate maturation schemes, with comparisons to traditional aging profiles—a start has been made here.9 Furthermore, Kew, et al.98 point out the need for fingerprinting of spirits—providing a unique chemical fingerprint of each sample. Technologies have advanced to more readily provide that fingerprint, and this will be useful in product development and aging regime comparisons. However, as stated elsewhere, it will still come down to flavor perceptions by the human senses to dictate the winners.
Distillers often ask for a determination of more components of spirits than necessary, both the identity and concentration of compounds. What it boils down to is a core set of flavor compounds (the base) and to the incredible, though subtle, influence and interplay of the hundreds to thousands of congeners with solvent and each other, all present at less than 1% of the total mass of a spirit—all presenting themselves in harmony (or discord) that indicate how well the spirit has been made and matured. These base compounds are the most flavor active and will serve as the markers of maturation from a chemical profiling aspect—sensory evaluation will take care of the rest—whether the product is in balance, harmony or has the right amplitude (generic hedonistic sensory terms). In the end, this reminds us to be patient and observe the rule noted earlier: The centuries-old formula: alcohol + oak + time = deliciousness!
As stated earlier in the quote by Mosedale, barrel aging involves many disciplines with much, much more research needed to unravel the complexities of maturation.15 This will apply to the use of wood chips, wood dust and wood spirals and to the application of many novel and even eccentric inventions and processes to try to beat Mother Nature and Father Time. The research that is being undertaken “seeks to develop understanding as to which chemical compounds are the most odor active contributors to estery and mature/woody-aged characters in malt whiskey, tequila and bourbon, and enable us to clarify the flavor interactions between these characters in each of the spirits.”8, 9, 76, 78 The challenges faced in measuring the taste and mouthfeel characteristics in distilled spirits were discussed and summarized recently.77 The importance of the topic is also noted from the fact that a third of the time allotted to distilled spirits talks at the recent Worldwide Distilled Spirits Conference (2017) was related to sensory evaluation and flavor chemistry.
The final reference here is a chapter dealing with questions and answers to current challenges and future solutions for maturation, an international spirits symposium workshop report.99 Rapid maturation of Scotch is still being questioned by the international whiskey industry. One question dealt with steel-tank-aged wines matured with oak chips, shavings or staves supplying wood characters and the potential extension to the cask-matured spirit sector. There was some potential for use of such wood products though “certain reactions, influenced by oxygenation over time, do not occur in these systems.” Other comments suggested selecting the right kind of wood and making sure it has been seasoned and toasted correctly. Finally, the esteemed Scottish whiskey researcher Dr. Connor noted that “if you were trying to achieve similar maturation as that given to Scotch Whisky by cask maturation, one of the problems would be that Scotch is traditionally matured in used wood, so getting the right kind of chips to achieve a similar profile would be difficult.” Such a product would not be allowed for sale under European Union rules as Scotch. This leads us to state that further research will be needed with multi-use barreling to see what happens, though this would be over several decades—not what rapid-maturists would want to hear.
The ultimate proof of any maturation system or process will be in the pudding—the true, authentic taste evaluation by highly trained sensory experts, those who can truly distinguish new from old. The cask may yet reign supreme—only time (and a lot of it) will tell. Let the new research and games begin!
References
[All linked references were last accessed in October 2017 and should be active]
1. Mosedale, J.R. and Puech, J-L. (1998). Trends in Food Science & Technology. 9; 95-101.
2. Le Floch, A., Jourdes, M. and Teissedre, P-L. (2015). Carbohydrate Research. 417; 94-102.
3. Mitchell, A.P. (2006). http://www.capewineacademy.co.za/dissertations/AndrewMitchell_CWM_Thesis.pdf
4. Oberholster, A., Elmendorf, B.L., Lerno, L.A., King, E.S., Heymann, H., Brenneman, C.E. and Boulton, R.B. (2015). Food Chem. 173: 1250-8.
5. Quesada Granados, J., Merelo Guervós, J.J., Oliveras López, M.J., González Peñalver, J., Olalla Herrera, M., Blanca Herrera, R. and López Martinez, M.C. (2002). J. Agric. Food Chem. 50 (6); 1470-1477.
6. Rogerson, L.E. (2016). Master’s Thesis, University of Tennessee. http://trace.tennessee.edu/utk_gradthes/3804/
7. Putman, R. (2016). Brewer and Distiller International. November 2016; 36-44.
8. Gonzalez-Robles, I. & Cook, D. (2014). University of Nottingham Web-linked Conference Poster.
9. Gonzales-Robles, I.G. and Cook, D.J. (2017). University of Nottingham Web-linked Poster. [Last accessed, October 2017.]
10. Kontoudakis, N., Gonzalez, E., Gil, M., Esteruelas, M., Fort, F., Canals, J.M. and Zamora, F. (2011). J. Agric. Food Chem. 59; 1974-1984.
11. Kilmartin, P.A. (2010). Adv. Food. Nutr. Res. 61; 149-86.
12. Reazin, G.H. (1981). Am. J. Enol. Vitic. 32 (4); 283-289.
13. Baldwin, S., Black, R.A., Andreasen, A.A. and Adams, S.L. (1967). J. Agric. Food Chem. 15 (3); 381-385.
14. Mosedale, J.R. (1994). Ph.D. Thesis. Trinity College and Oxford Forestry Institute, University of Oxford.
15. Mosedale, J.R. (1995). Forestry. 68 (3); 203-230.
16. Mitchell, B. (2015). Brewer and Distiller International. May 2015; 22-27.
17. Nykanen, L. and Lehtonen P. (Eds.) (1984). Flavor Research of Alcoholic Beverages: Instrumental and Sensory Analysis. The Alko Symposium, Helsinki, Finland, 1984.
18. Adams, P. (2012). https://www.popsci.com/technology/article/2011-12/scientific-ways-make-whiskey-taste-older-faster
19. Bell, E. (2016). https://vinepair.com/wine-blog/a-shortcut-to-aged-whiskey-product-testing-the-oak-barrel/
20. Bobbe, A.B. (1962). http://www.google.com.pg/patents/US3021780
21. Bryson, L. (2017). https://www.thedailybeast.com/extreme-whiskey-barrel-aging.
22. Carmody, W.H. and Hochwalt, C.A. (1936). https://www.google.com/patents/US2027099
23. Chamberlain, C. (2014): http://www.foodrepublic.com/2014/05/07/is-lightning-aging-the-future-of-the-bourbon-industry-god-save-the-industry/.
24. Chris and Darren (2015). https://bottomofthebarrelbourbon.com/2015/04/10/better-aging-through-chemistry/
25. Cowdery, C. (2015). http://chuckcowdery.blogspot.com/2015/08/the-accelerated-aging-challenge.html
26. Emen, J. (2016). https://thewhiskeywash.com/whiskey-styles/american-whiskey/rapid-aging-whiskey-technology-game-changer-gimmick/
27. Fain, J.M. and Snell, F.D. (1934). Chem. Eng. News. 12 (7); 120-121.
28. Garland, J. (2017). The GROWLER. https://growlermag.com/fooling-father-time-rapid-aging-future-craft-whiskey/
29. Goldfarb, A. (2015). http://www.esquire.com/food-drink/drinks/a35979/rapidly-aged-whiskey-primer/
30. Grossmann, J. (2013). https://www.inc.com/magazine/201306/john-grossman/whiskey-cleveland.html
31. Greenblatt. A. (2013). http://www.npr.org/sections/thesalt/2013/05/02/180661986/how-a-distillery-ages-bourbon-in-days-not-years.
32. Herrington, C. (2015). http://www.npr.org/sections/thesalt/2015/05/17/407064152/south-carolina-distiller-promises-to-make-kentucky-liquor-quicker
33. Independent Stave Co and Woodinville Whiskey. (2017). http://www.americancraftspirits.org/wp-content/uploads/2016/05/Flavor-Profiles-Barrel-Presentation.pdf
34. Knapp, A. (2013). https://www.forbes.com/sites/alexknapp/2013/05/29/cleveland-whiskey-ages-bourbon-in-one-week/#14bce9e065cd
35. Lix, T.S. (2013). https://www.google.com/patents/US20130149423
36. Lost Spirits (2015). Lost Spirits: The Model 1—21st Century Oak Aging. White Paper. April 2015.
37. Marsteller, A. (2015). https://www.liquor.com/articles/experimental-spirits-aging-techniques/#gs.DT9dRpw
38. McLafferty, C. (2016). https://arstechnica.com/science/2016/07/the-scientific-arms-race-to-age-our-whiskey/
39. Minnick, F. (2013). https://www.scientificamerican.com/article/whiskey-makers-break-tradition-to-make-new-flavors/ Scientific American.
40. Niazi, S.K. (2016). https://www.google.com/patents/US20160376538
41. Nickol, G.B. (1954). https://www.google.com/patents/US2807547
42. Pomranz, M. (2017). Food & Wine. http://www.foodandwine.com/news/distillery-just-got-patent-process-ages-whiskey-in-week
43. Quora Contributor (2016). http://www.slate.com/blogs/quora/2016/04/21/is_oak_the_only_acceptable_wood_for_aging_whiskey.html.
44. Reiman, C.K. (1938). https://docs.google.com/viewer?url=patentimages.storage.googleapis.com/pdfs/US2132435.pdf
45. Remy, F.G. II, Delmore, J. P. and Buske, W.E. (2004). https://www.google.com/patents/US6703060
46. Rogers, A. (2014). WIRED. https://www.wired.com/2014/05/making-time-go-faster-for-aged-booze/
47. Sachs. T. (2015). HuffPost The Blog. https://www.huffingtonpost.com/tony-sachs/post_9243_b_6980700.html
48. Singleton, V.L. (1962). Hilgardia. 32 (7); 319-392.
49. The Staff (2017). Beverage Dynamics. http://beveragedynamics.com/2017/04/19/lost-spirits-awarded-patent-for-high-tech-maturation-techniques/
50. Carmody, W.H. and Hochwalt, C.A. (1936). Aging of Whiskey. US Patent: US 2027099 A. https://www.google.com/patents/US2027099
51. Brady, H. (2016). http://www.nationalgeographic.com/people-and-culture/food/the-plate/2016/07/whiskey-distilling-production-entrepreneurs-market-science/
52. Liebmann, A.J. and Rosenblatt, M. (1943). Industrial and Engineering Chemistry. 35 (9); 994-1002.
53. Liebmann, A.J. and Scherl, B. (1949). Industrial and Engineering Chemistry. 41 (3); 534-543.
54. Baldwin, S. and Andreasen, A.A. (1974). Journal of the AOAC. 57 (4): 940-950.
55. Reazin, G.H., Baldwin, S., Scales, H.S., Washington, H.W. and Andreasen, A.A. (1976). Journal of the AOAC. 59 (4); 770-776.
56. Boruff, C.S. and Rittschof, L.A. (1959). J. Agric. Food Chem. 7 (9); 630-633.
57. Piggott, J.R., Conner, J.M., Paterson, A. and Clyne, J. (1993). Int. J. of Food Science and Technology. 28; 303-318.
58. Schoeneman, R.L., Dyer, R.H. and Earl, E.M. (1971). Journal of the AOAC. 54 (6): 1247-1261.
59. Reazin, G.H. (1983). In: Current Developments in Malting Brewing and Distilling [F. G. Priest, and I. Campbell. Eds.] Aviemore Conference 1982. Institute of Brewing, London. pp 205-224.
60. Delahunty, C.M., Conner, J.M., Piggott, J.R. and Paterson, A. (1993). J. Inst. Brew. 99 (6); 479-482.
61. Spedding, G. (2017a). Artisan Spirit 18 – Spring 2017; 98-102.
62. Spedding, G. (2017b). Artisan Spirit 19 – Summer 2017; 65-69.
63. Panossian, A., Mamikonyan, G., Torosyan, M. Gabrielyan, E. and Mkhitaryan, S. (2001). Anal. Chem. 73; 4379-4383.
64. Tanaka, H., Kitaoka, T., Wariishi, H. and Ishihara, C. (2002). J. Food Sci. 67 (8); 2881-2884.
65. Gallager, M., Kolachov, P. and Willkie, H. (1942). Ind. Engineering Chemistry. 34 (8); 992-995.
66. Boothroyd, E.J., Linforth, R.S.T. and Cook, D.J. (2012). J. Agric. Food Chem. 60; 9959-9966.
67. Conner, J. (2014). Maturation. In: Whisky: Technology, Production and Marketing (Second Edition). Inge Russell and Graham Stewart (Editors). Elsevier/Academic Press. 199-220
68. Conner, J.M., Paterson, A. and Piggott, J.R. (1992). J. Sci. Food Agric. 60 (3); 349-353.
69. Conner, J.M., Paterson, A. and Piggott, J.R. (1994a). Agglomeration of ethyl esters in model spirit solutions and malt whiskies. J. Sci. Food Agric. 66 (1); 45-53.
70. Conner, J.M., Paterson, A. and Piggott, J.R. (1994b). J. Agric. Food Chem. 42; 2231-2234.
71. Conner, J. M., Paterson, A. and Piggott, J.R. (1999). J. Sci. Food Agric. 79 (7); 1015-1020.
72. Conner, J.M., Birkmyre, L., Paterson, A. and Piggott, J. (1998). J. Sci. Food. Agric. 77: 121-126.
73. Conner, J.M., Paterson, A., Birkmyre, L. and Piggott, J.R. (1999). J. Inst. Brew. 105 (5); 287-291.
74. Conner, J.M., Paterson, A., Piggott, J.R. and Whateley, T.L. (1998). J. Agric Food Chem. 46 (4); 1292-1296.
75. Piggott, J.R., Conner, J.M., Clyne, J. and Paterson, A. (1992). Sci. Food Agric. 59; 477-482.
76. Overbosch, P., Afterof, W.G.M. and Haring, P.G.M. (1991). Food Reviews International. 7 (2); 137-184.
77. Jack, F. (2015). In: 5th Worldwide Distilled Spirits Conference Proceedings. [I. Goodall, R. Fotheringham, D. Murray, R.A. Speers, and G.M. Walker, Editors]. Institute of Brewing and Distilling. Chapter 38: 183-188.
78. Macatelli, M., Paterson, A. and Piggott, J.R. (2008). In: Distilled Spirits: Production, Technology and Innovation. (J.H. Bryce, J.R. Piggott and G.G. Stewart, Eds.) Nottingham University Press. Chapter 22; 151-158.
79. Karlsson, B.C.G. and Friedman, R. (2017). Scientific Reports. 7, Article number 6489; 1-9. https://www.nature.com/articles/s41598-017-06423-5
80. du Toit. W.J. (2010). In: Managing Wine Quality. Vol. 2. Oenology and Wine Quality. (A.G. Reynolds, Ed.). Woodhead Publishing. Chapter 8; 226-254.
81. Cottrell, T. (2004). https://www.winebusiness.com/wbm/?go=getArticleSignIn&dataId=30476
82. del Carmen Llaudi, M., Canals, R., Gonzalez-Manzano, S., Canals, J.M., Santos-Buelga, C. and Zamora, F. (2006). J. Agric. Food Chem. 54; 4246-4252.
83. Quideau, S. (Ed.) (2009). Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols. World Scientific Publishing Co.
84. Tao, Y., Garcia, J.F. and Sun, D.W. (2014). Crit. Rev. Food. Sci. Nutr. 54 (6); 817-35.
85. Waterhouse, A.L., Sacks, G.L. and Jeffery, D.W. (2016). Understanding Wine Chemistry. Wiley.
86. Pons, A., Prida, A., Verdier, B., Darriet, P. and Dubourdieu, D. (2015). Practical Winery and Vineyard. Feb. 2015; 59-66. http://seguinmoreaunapa.com/pdfs/PWV_Barrel_Alternatives_Supply_Oxygen.pdf
87. Waters, D. (2012). National Wine and Grape Industry Center. https://www.csu.edu.au/__data/assets/pdf_file/0005/455198/NWGIC-fs5-micro-oxygenation.pdf
88. Elias, R.J. and Waterhouse, A.L. (2010). J. Agric. Food Chem. 58: 1699-1707.
89. Atanasova, V., Fulcrand, H., Cheynier, V. and Moutounet, M. (2002). Analytica Chimica Acta. 458; 15-27.
90. Quinn, M.K. and Singleton., V.L. (1985). Am. J. Enol. Vitic. 36 (2); 153-155.
91. Strlic, M. Radovic, T., Kolar, J. and Pihlar, B. (2002). J. Agric. Food Chem. 50; 6313-6317.
92. Vivas, N. and Glories, Y. (1996). Am. J. Enol. Vitic. 47 (1); 103-107.
93. Hemingway, R.W. and Karchesy, J.J. (Eds). (1989). Chemistry and Significance of Condensed Tannins. Plenum Press, NY.
94. Copeland, R.A. Enzymes. (1996). Enzymes: A Practical Introduction to Structure, Mechanism and Data Analysis. Wiley-VCH.
95. Cornish-Bowden, A. (1995). Fundamentals of Enzyme Kinetics (Revised Edition). Portland Press, London.
96. Price, N.C. and Stevens, L. (1989). Fundamentals of Enzymology. (Second Edition). Oxford University Press.
97. Ramey, D.D. and Ough, C.S. (1980). J. Agric. Food Chem. 28 (5); 928-934.
98. Kew, W., Goodall, I., Clarke, D. and Uhrin, D. (2016). J. Am. Soc. Mass Spectrom. Published online 17 October 2016.
99. Goodall, I. (Chair) (2015). Current challenges and future solutions for maturation: workshop report. In: Proceedings of the 5th Worldwide Distilled Spirits Conference.
The full titled citations noted here can be found at the Distiller website. In addition to these references, many more were consulted in preparation of the manuscript or were listed within notes written by the author over the past two years. A fuller listing can be made available upon request. A library of such information should be available for the distiller to draw upon, and discussions are underway with various distilling groups to provide a central resource for such information. Stay tuned.