Of all the things in a distillery that could spark (pun intended) a fire or explosion, electrical systems and equipment are at the top of the list, followed closely by static electricity and open flames. The solution to the risk of fire or explosion (actually deflagration) is simple physics. That’s right, “simple” physics. While the complex mathematics used in the study of physics is anything but simple, the basic principles are. If we can understand the basic concepts of the process of combustion, we can begin to gain an intuitive understanding of the laws of physics that dictate how and why things burn and what effects combustion can have under various circumstances.
Detonation vs. Deflagration
A detonation is supersonic. A deflagration is subsonic. Both can have an explosive effect. When considering alcohol, we are dealing with a deflagration, which is the rapid burning of a fuel-air mixture. In this case, the fuel is ethanol, either atomized or vaporized. Atomized liquid is a very fine mist where the material is still in a liquid form. Vaporized liquid has undergone a phase change from liquid to vapor. It has exceeded its boiling point. This happens inside our stills between the pot and condenser.
Flamethrower vs. Potato Gun:
conditions that lead to an explosive effect

Confinement is the key. When I was younger, a friend of mine (of course it was my friend doing this) applied a lighter to the stream coming from the nozzle of his mother’s aerosol hair spray can. The flammable liquid in the can was atomized into a mist. The fuel mixed with the air and burned. Dangerous enough if you or some other fuel is in the path of the fire. In the potato gun analogy, the same rapid burning of a fuel-air mixture is confined. The confinement is what causes the explosive effect. As the confined burning fuel-air mixture expands, the pressure is relieved via the path of least resistance and the movable potato is launched. In Figure 1, you see a still that has become a “flamethrower.” Trouble is, the mist has not yet been ignited. If it had been, the resultant damage would have actually been less dramatic than it was. Instead, the fuel filled the room, like the chamber of the potato gun, before it was ignited. The confinement of the room resulted in an explosive effect. We don’t want flamethrowers or potato guns in our distilleries. But we especially do not want the potato gun analogy because, in a distillery fire, you are inside the combustion chamber of the potato gun. The solution to this is simple physics. No, it is not to have candles around in case of a vapor leak to burn it off the way methane vents are burned at landfills. The solution, in addition to keeping ignition sources away from potential fuel sources, is to remove the fuel and fuel-air mixture, assuring that it is not possible for fuel levels to be high enough for combustion.
Basic Physics
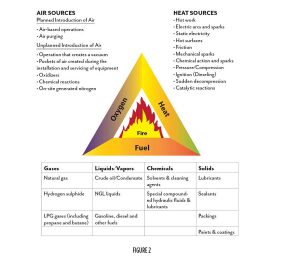
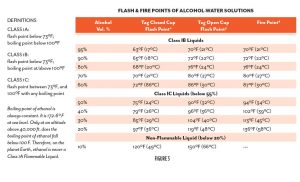
For combustion to take place, all three components of the fire triangle shown in Figure 2 must be present and present at the necessary level and in the necessary relative quantities. The heat source must be greater than the flash point, and greater than or equal to the fire point of the fuel substance. The flash points and fire points of ethanol-and-water mixtures of varying concentration are shown in Figure 3. Notice the difference between the flash point, where sustained combustion is not possible, and the fire point, where it is. The temperature spread narrows as the concentration of ethanol increases. At sea level, less than 20% ethanol has no fire point. This is what FM Global refers to, in their data sheets, as “non-ignitable.” Understanding the increasing temperature differential between flash point and fire point as ethanol concentrations are reduced is important when considering the relative risk difference between 1C flammable liquids (under 55% ABV at sea level) and the higher ethanol concentrations in 1B flammable liquids. The codes do not address this. However, when considering specific applications and alternative design methods, this gradient should be taken into consideration.
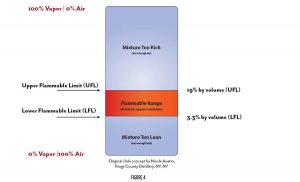
For combustion to take place, the fuel-air mixture must be within the flammable range, greater than the LFL (Lower Flammable Limit) but less than the upper flammable limit. The flammable range of ethanol is shown in Figure 4. We can’t remove the oxygen in our distilleries. We sort of need that to breathe. But we can eliminate the heat source and we can limit the fuel so as to assure concentrations remain below 25% of the LFL. This is the standard of safety set forth in the building, fire and flammable liquids codes.
The Electric Sombrero of Death
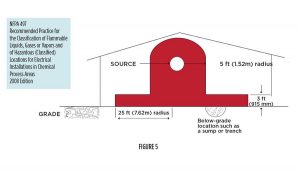
The Electric Sombrero of Death shown on page 16 is an artistic rendering of the concepts and code basis for establishing the electrically classified area relative to a potential source of ethanol vapor release. It is based on one of a series of diagrams found in NFPA 497, “Recommended Practice for the Classification of Flammable Liquids, Gases, or Vapors and Hazardous (Classified) Locations for Electrical Installations in Chemical Process Areas.” See Figure 5. Keep electrics, potential static discharges, and open flames out of the Sombrero and everything is cool. This eliminates heat in the fire triangle. Penetrate the Sombrero border and you can expect to spend tenfold on wiring that meets the classification standards. Think of anything, especially the still head, with ethanol over 20% ABV, as a potential source of vapor release.
Math vs. Money
Inevitably, there will be some feature of our distilleries that absolutely requires electrics to breach the perimeter of the Sombrero. Examples include lighting in sight glasses, rummaging motors not ordered to classified specifications, condensate return pumps, and electrically heated or direct-fired stills. This is where the math vs. money idea comes in. While all the codes require us to respect the classified area represented by the Sombrero, the fire code and the flammable liquids code (referencing NFPA 497) both permit us to eliminate the classified area, provided we can demonstrate to the AHJ (Authority Having Jurisdiction), i.e., the fire and/or building code official, that it is not possible to exceed 25% of the LFL. Ventilation is the key to assuring that if you have a vapor leak that is ignited, at worst, it becomes a flamethrower and you are not placed inside a potato gun. Consider the operation of an internal combustion engine in your car. Flood the engine, too much fuel, no combustion; not enough fuel, too much air, under the LFL, no combustion. The calculations to demonstrate that exhaust is removing any ethanol vapor faster than it can build up in excess of 25% of the LFL do require some math and do cost something. But that cost is usually far less than the cost of providing class- and division-compliant electrical systems.
Sources of Ethanol Vapor
There are three usual potential sources of ethanol vapor release that must be considered. Normally open containers are not addressed here because most distilleries have no desire to keep things like maceration tanks open to the air, as evaporating ethanol is like evaporating money.
1. The still is usually the driving factor. To size an exhaust and ventilation system to compensate for the worst-case scenario, a breach in a part of the still where ethanol is in its vapor state, the following information is required.
a. Volume and ABV of ethanol in.
b. Volume and ABV of ethanol out.
c. Distillation time.
2. Spill control and secondary containment are required where MAQs (Maximum Allowable Quantities) per code are exceeded. Generally, if you exceed the MAQs the space also becomes an H-2 or H-3 hazardous occupancy. If the method of secondary containment could result in a puddle of alcohol in the containment area, ventilation must be sized to remove evaporating ethanol vapor faster than it can build up to 25% of the LFL. The following information is required to size the ventilation system for this scenario.
a. Maximum design volume of the puddle, which is equal to the maximum size vessel within the containment area.
b. Surface area of the containment
puddle.
c. Regional data such as local average barometric pressure and elevation.
d. Maximum room temperature under normal operating conditions.
3. We all know about the angel’s share. The amount of ethanol that evaporates from stored barrels will dictate the ventilation requirements for cask storage areas. The good news is that this ventilation rate is very, very low. Declassification ventilation in barrel rooms usually does not exceed the very low code-mandated ventilation rates for cask storage. These rates are much lower than required in hazardous occupancies (where MAQs are exceeded) or to electrically declassify for still breach or spill puddle. The following data is needed to design ventilation systems for cask storage areas to maintain ethanol concentration under 25% of the LFL.
a. Number and volume of barrels.
b. Area of storage.
c. Average ABV of stored ethanol.
d. Historic angel’s share loss per TTB reporting for established distilleries or regional average for new facilities.
Completing these calculations and designing the ventilation system is somewhat beyond “simple physics.” Nonetheless, taking the time and incurring the relatively (when compared to installing classified location compliant electrics) small cost to do the math, generally results in a safer and more financially friendly distillery.
Conclusion
Safe distillery design comes down to two simple practices:
Keep electrics out of the classified area or Electric Sombrero of Death.
Provide ventilation (exhaust and makeup air) in the correct location sized sufficiently to eliminate the classified area and assure ethanol vapor concentrations are maintained at less than 25% of the LFL.
015 IBC 202 – DETONATION. An exothermic reaction characterized by the presence of a shock wave in the material, which establishes and maintains the reaction. The reaction zone progresses through the material at a rate greater than the velocity of sound. The principal heating mechanism is one of shock compression. Detonations have an explosive effect.
015 IBC 202 – DEFLAGRATION. An exothermic reaction, such as the extremely rapid oxidation of a flammable dust or vapor in air, in which the reaction progresses through the unburned material at a rate less than the velocity of sound. A deflagration can have an explosive effect.
015 IBC 202 – EXPLOSION. An effect produced by the sudden violent expansion of gases, which may be accompanied by a shock wave or disruption, or both, of enclosing materials or structures. An explosion could result from any of the following:
a. Chemical changes such as rapid oxidation, deflagration or detonation, decomposition of molecules and runaway polymerization (usually detonations).
b. Physical changes such as pressure tank ruptures.
c. Atomic changes (nuclear fission or fusion).
015 IBC 202 – FLASH POINT. The minimum temperature in degrees Fahrenheit at which a liquid will give off sufficient vapors to form an ignitable mixture with air near the surface or in the container, but will not sustain combustion. The flash point of a liquid shall be determined by appropriate test procedure and apparatus as specified in ASTM D 56, ASTM D 93 or ASTM D 3278.
015 IBC 202 – FIRE POINT. The lowest temperature at which a liquid will ignite and achieve sustained burning when exposed to a test flame in accordance with ASTM D 92.
M Global is an insurance company that also conducts tests on various building and equipment components, much like UL (Underwriters Laboratory). Its previous name was Factory Mutual.
015 IBC 202 – LOWER FLAMMABLE LIMIT (LFL). The minimum concentration of vapor in air at which propagation of flame will occur in the presence of an ignition source. The LFL is sometimes referred to as “LEL” or “lower explosive limit.”
015 IFC 5703.1.1 – Classified locations for flammable liquids. Areas where flammable liquids are stored, handled, dispensed or mixed shall be in accordance with Table 5703.1.1. A classified area shall not extend beyond an unpierced floor, roof or other solid partition. The extent of the classified area is allowed to be reduced, or eliminated, where sufficient technical justification is provided to the fire code official that a concentration in the area in excess of 25 percent of the lower flammable limit (LFL) cannot be generated.
015 NFPA 30-A.7.3.3 – For additional information, see NFPA 497, Recommended Practice for the Classification of Flammable Liquids, Gases, or Vapors and of Hazardous (Classified) Locations for Electrical Installations in Chemical Process Areas.
2012 NFPA 497-5.4.1 – Experience has shown that the release of ignitable mixtures from some operations and apparatus is so infrequent that area classification is not necessary. For example, it is not usually necessary to classify the following locations where combustible materials are processed, stored, or handled:
a. Locations that have adequate ventilation, where combustible materials are contained within suitable, well maintained, closed piping systems
b. Locations that lack adequate ventilation, but where piping systems are without valves, fittings, flanges, and similar accessories that may be prone to leaks
c. Locations where combustible materials are stored in suitable containers
d. Locations where the use of combustible liquids, or flammable liquids or gases, will not produce gas or vapor sufficient to reach 25 percent of the lower flammable limit (LFL) of that combustible material