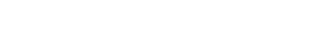While yeast are a fungus, they are surprisingly close genetically to animals (including humans). Just like us, yeast need a vast variety of energy and nutrient sources to survive and grow. They thrive on oxygen and release carbon dioxide.
While there are thousands of types of yeast and hundreds of subtypes of saccharomyces (with many more yet to be discovered), the one used most for alcohol production is Saccharomyces cerevisiae. Saccharomyces is Latin for sugar fungus. Cerevisiae is Latin for beer and is the origin for the Spanish word cerveza. There are many different strains of Saccharomyces cerevisiae which are distinguishable phenotypically — by their fermentation behavior. For example, the extent it will ferment different sugars, the amount of oxygen it needs, the amount and type of by-products it produces, or its behavior in suspension. Different strains can also be differentiated genotypically by their DNA.
Many distillers use generic yeast based on total ethanol production or speed of fermentation. What is less commonly discussed are the other compounds that yeast produce that can contribute to the development of a wide range of different flavors — some desirable, some less so. Yeast is an underutilized variable in creating flavor in the distillery, and the rest of this article will discuss ways distillers can manipulate fermentation to change the character of their products.
Yeast By-Products
Illustration 1 is a visual representation of the compounds yeast are able to break down and release back into the fermentation media.
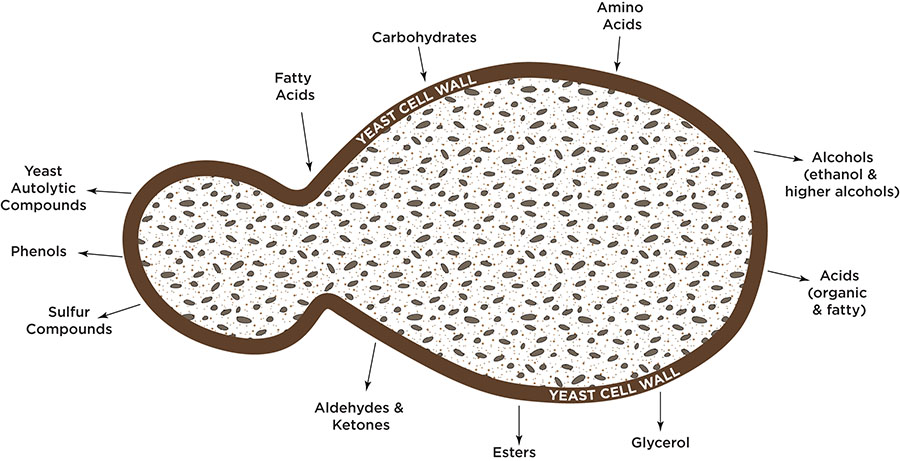
Yeast bring fatty acids, carbohydrates, and amino acids in through their cell walls. These compounds can then go through a number of different pathways within the yeast cell. Some pathways are known and have been studied, but many pathway mechanisms and triggers are unknown. The yeast can secrete organic and fatty acids, esters, aldehydes, ketones, phenols, autolytic compounds, glycerol, and of course alcohols back into the fermentation broth. Various combinations of these compounds create different flavors and aromas in the final product. Some of these compounds have a positive impact while others, in excess, can cause unwanted flavors and aromas. Some examples of by-products of fermentation can be seen in the table below.
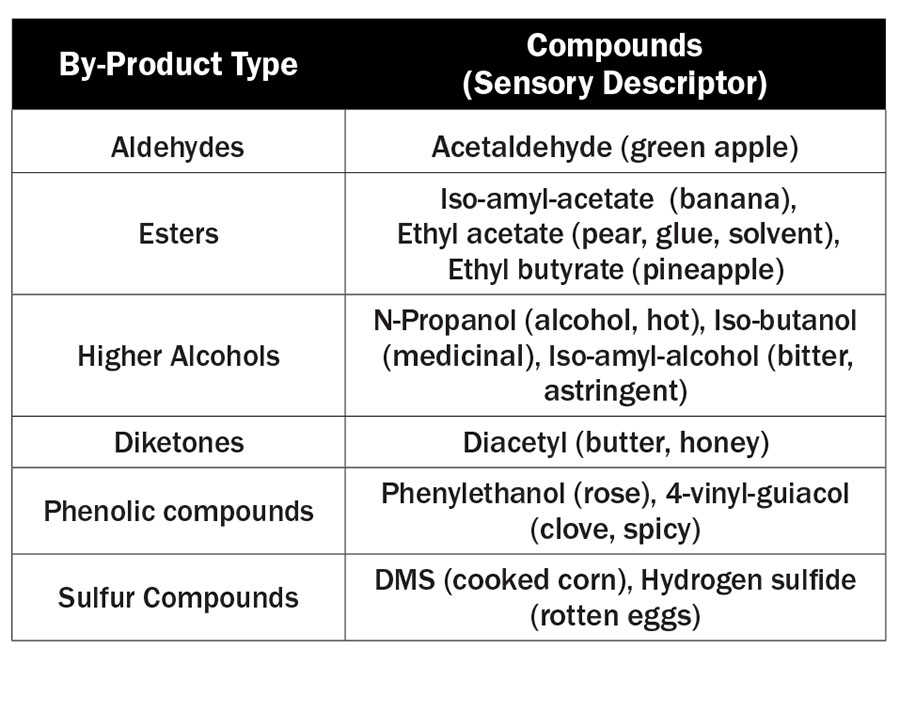 The two main compound classes that can be easily manipulated in fermentation are esters and higher alcohols. Esters are most responsible for fruity aromas, while higher alcohols contribute to the aging potential of a distillate. Both are formed via the Ehrlich pathway, which is outlined in Illustration 2.
The two main compound classes that can be easily manipulated in fermentation are esters and higher alcohols. Esters are most responsible for fruity aromas, while higher alcohols contribute to the aging potential of a distillate. Both are formed via the Ehrlich pathway, which is outlined in Illustration 2.
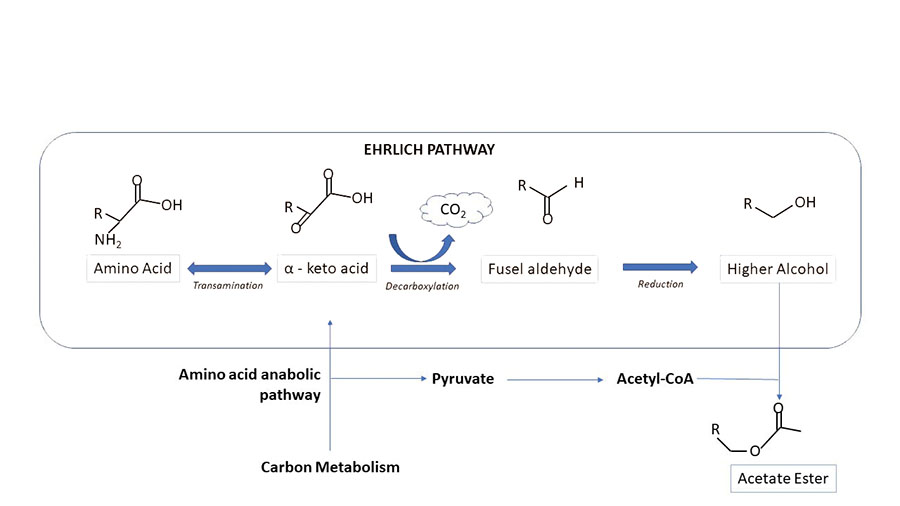
As you can see, alcohols, given the right time and environment, can eventually become esters. Organic acids inhibit the final step of the Ehrlich pathway. Therefore, an increased percentage of organic acids reduces ester formation.
Each unique yeast strain may respond differently based on its environment and available nutrients, but there are several main parameters to vary to create your unique flavor profile.
Oxygen and Aeration
For yeast to survive, they need energy in the form of ATP (adenosine triphosphate) created either through aerobic respiration or anaerobic fermentation. Yeast need oxygen to aerobically respire and duplicate. Yeast duplicate by budding. Each mother cell creates one daughter cell at a time, as shown in Illustration 3.
Yeast also need oxygen to synthesize new cell walls. So, if there is no oxygen present, yeast cannot duplicate. To survive after oxygen depletion, they switch to fermenting. Growing yeast ferment up to 30 times faster than non-growing yeast, so special care should be taken to ensure the yeast are growing prior to pitching into fermentation either by propagation or yeast rehydration. More initial oxygen in the fermentation broth leads to more growth, which tells the yeast to go down certain pathways creating a certain set of compounds. Studies at VLB Berlin showed that increased aeration caused increased levels of higher alcohols. The opposite was true for esters. A decrease in oxygen levels in the beginning of fermentation led to an increase in ester production by up to 40%.
Temperature
Assuming a temperature-controlled fermentation tank, temperature is an easy way to manipulate pathways in the yeast cell. Yeast cells grow the best at 30°C (86°F). This may surprise some readers, as many fermentations happen a bit lower at 20–25°C (68–77°F). In general, higher temperatures are going to support more yeast growth. As we saw earlier, more yeast growth is tied to higher formation of higher alcohols. In terms of ester production, higher temperatures lead to a decrease in ester production.
There are limits to the ranges you should employ. Yeast will ferment very slowly at severely low temperatures, and if the temperature is exceedingly high, the yeast can be heat shocked and die prematurely. It is important to consult your yeast supplier to obtain the lethal temperature ranges for your yeast strain.
Starting Gravity and Sugar Profile
Starting or original gravity refers to the amount of sugars present in the fermentation broth in the beginning of fermentation. High sugar concentrations can only be handled by certain types of yeast. Wine yeast, for example, are accustomed to sugar concentrations up to 26 Brix or 1.11 specific gravity, while most beer yeasts would become stressed in these high sugar environments. Within the safe range, however, flavor compounds will vary with gravity. Higher starting gravities lead to more production of higher alcohols and more esters.
Types of sugars also affect the flavor compounds created in fermentation. Sugars vary in chemical structure and thus will alter pathways in yeast cells. Fermentations with a higher level of simple sugars like glucose and fructose will result in more esters than mashes with longer-chain sugars.
Pitching Rate
Yeast producers suggest a range of suitable pitching rates, and these ranges should not be ignored. Not pitching enough yeast can lead to slow or stuck fermentations. A good rule of thumb is 1 million cells per milliliter per degree Plato. Slightly increasing or decreasing from this value can cause variation in flavor compounds. Cell growth decreases as pitching rate decreases, especially when the pitching rate is more than needed in the fermentation. So, a smaller pitching rate will induce higher levels of higher alcohols and higher levels of esters.
Yeast Type
Yeast type has a large effect on the amount and type of esters produced, among other flavor and aroma compounds. Keeping all other variables the same, if you pitched with two different yeast strains, you would have two entirely different end products. Sulfur compounds that yeast produce are also dependent on yeast strain. Some strains do not create sulfur at all, while others are known to create significant amounts of sulfur under stress. Strains can also be characterized as phenolic off-flavor positive (POF +) or negative (POF -). POF + strains have the ability to transform phenolic acids into other phenolic compounds. One great example of POF + yeasts are German hefeweizen yeast strains, which convert ferulic acid in the grain into 4-VG (clove, spicy) and are responsible for isoamyl acetate (banana) esters. Without the use of this strain in brewing, hefeweizens would not have their traditional character.
Nutrients
Yeast nutrients include free nitrogen, vitamins, and metals. Yeast require nitrogen to metabolize sugars into alcohol. No or extremely low levels prevent fermentation from happening at all. Lower levels of free amino nitrogen lead to decreased yeast growth and consequently lower levels of higher alcohols.
Zinc is a very important metal for alcohol metabolism. The pathway from glucose to ethanol is multistep, but the last step is the conversion of acetaldehyde into ethanol by the enzyme alcohol dehydrogenase. Zinc is required for this enzyme to function properly. Therefore, if a spirit has high levels of acetaldehyde, the fermentation likely had a lack of zinc available. Interestingly, the same enzyme is responsible for breaking down alcohol in the body, so if you tend to have severe hangovers after a night out, try taking a zinc supplement before going to bed. It may make you feel better in the morning.
Head Pressure
Head pressure is referred to as the amount of pressure on the yeast. It is based on the total amount of liquid being stored in the vessel in a certain area which is applying a certain amount of force. A horizontal vessel holding the same amount of liquid would have less head pressure on yeast than a vertical vessel holding the same amount of liquid. This is because the weight of the liquid is spread out more in a horizontal vessel than in a vertical vessel. Higher head pressure on the yeast results in decreased yeast growth and consequently lower levels of higher alcohols. Decreased head pressure leads to higher levels of esters. This phenomenon is widely leveraged in the brewing industry. Horizontal fermenters are often used in lager production to promote more ester production. In fact, Anheuser Busch (1994) produced 50% of their product in horizontal fermenters and 50% in vertical fermenters. The horizontal fermenters produced 25% more total esters. Heineken only used horizontal fermenters to decrease head pressure on yeast and enhance ester production. Esters can also be increased by long secondary fermentations — that is, leaving the fermentation in the fermenter a day or two after it has reached terminal gravity. This gives more time for alcohols to convert to esters via the Ehrlich pathway.
There are many variables distillers can manipulate in the fermentation process to produce desired flavor and aroma compounds. It is important to think about the style and beverage type you are producing. In aged spirits, it may be desirable to have more congeners, aka more higher alcohols, to facilitate better barrel-aging reactions. Brandies, however, may benefit from higher esters to complement the fruity character. I recommend consulting your yeast supplier to experiment with types of yeast and fermentation environments tailored to your goals, products, and facility. Happy fermenting!
References
White, Chris. Zainasheff, Jamil. Yeast: The Practical Guide to Beer Fermentation. Boulder, Colorado, Brewers Publications, 2010.
Lea, A.G.H. Piggot, J.R. Fermented Beverage Production. Chapman & Hall, 1995.
Bamforth, Charlie. Scientific Principles of Malting and Brewing. American Society of Brewing Chemists, 2006.
Stewart, Graham. Brewing Intensification. American Society of Brewing Chemists, 2014.
Pitch Rate. Wyeast Lab. 2021. https://wyeastlab.com/resource/professional-pitch-rate/
Salas-Millan. Aznar Arantxa. Aguayo, Encarma. Revalorization of Melon By-Product to Obtain a Novel Sparkling Fruity-Based Wine. Foods. 2023.