Sometimes it’s good to go back to the basics. A refresher on a seemingly familiar topic can be… well, refreshing.
Mash chemistry is one of those topics that a lot of folks feel like they have a solid handle on. Seems easy, doesn’t it? Take starch-heavy grain, apply heat and water, infuse with a little patience and you should have yourself a fermentable sugar profile that your yeast can produce alcohol from.
If this all sounds simple that’s because on the surface it is. Of course, this seeming simplicity obscures a complicated set of chemical reactions worthy of a deeper understanding. Let’s take a look at mashing chemistry and what actually happens under the liquid hood. For some people this may be a bit of a refresher, but the goal is that you walk away with a more refined understanding of mashing principles. For others this material may feel entirely new, and hopefully, you’ll feel that much more confident when it comes time to tackle the mysteries of grain to glass. So, let’s dive in, shall we?
The Problem of Starch
Why can’t we simply take milled wet grain, add yeast and get alcohol? Hell, it works just fine with grapes and honey. Shouldn’t we be able to do the same thing with our barley and corn? That’d be nice, but unfortunately things just don’t work that way. The reason is that while grapes are composed of simple fermentable sugars, grains are composed of starch.
As far as the plant is concerned, starch is not there for us to reap its alcohol-producing potential. To the plant, starch serves as a form of energy storage that the embryo plant can use when it starts to germinate. Of course, we’re human beings, dammit, and there’s very little on this earth that someone hasn’t tried to ferment and get a buzz off of. At some point in our history of drunken befuddlement someone figured out that, if you add water and the right amount of heat to grain, the resulting liquid will taste sweet. Turn a few centuries’ worth of pages and we’ve realized that yeast exist to consume sugars from sweet-tasting things (including mashed grain) and produce alcohol as a by-product.
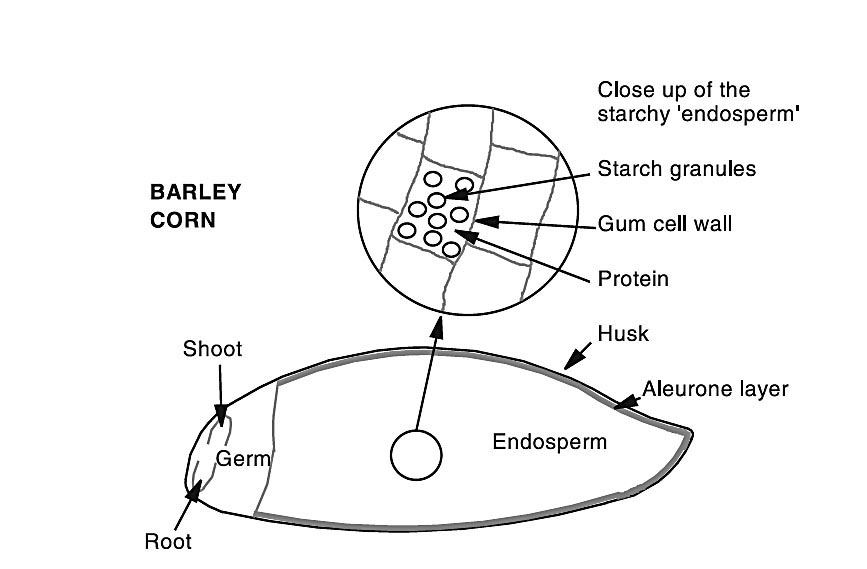
To understand how mashing works, it helps to take a closer look at what the inside of a grain kernel looks like. Figure 1 shows an anatomical drawing of a
barley kernel. Don’t worry if you use other grains instead of barley; many (but not all) of the structures of barley hold true for the other common whiskey grains. Note the large area taken up by the endosperm: This is where the starch is held.
Endosperm starch is bound up into microscopic granules. Different grains have different sizes and shapes of granules. In barley there are two types of starch granules, both basically spherical in shape. Type A granules are the larger of the two, usually about 20 µm in size. Type B granules are much smaller, generally ranging from 3–10 µm.
Now why is this important? In order for our mash to be successful we need water (we’ll see exactly why later) to be able to penetrate the inner depths of the starch granule to unfold and gelatinize it. Smaller granules are more compact than their bigger brothers and therefore don’t allow water to flow through quite as easily. This is why corn typically requires more energy to mash than other grains. Corn starch is primarily made up of smaller compact starch granules, making their gelatinization more difficult.
So, we know that we need to gelatinize the starch granules, essentially opening them up with water and forming a gel. The reason for that is we want better access to what composes the starch. Grain starch is composed of glucose molecules bound together in chains. These grain starch chains come in two types: amylose and amylopectin. Amylose is essentially a straight chain of glucose molecules, not too complicated, but it has the ability to form pretty dense starch granules, particularly in some varieties of corn. Amylopectin has a branched structure of glucose strands and for many grains makes up about 75% of the total starch content (see Fig. 2).
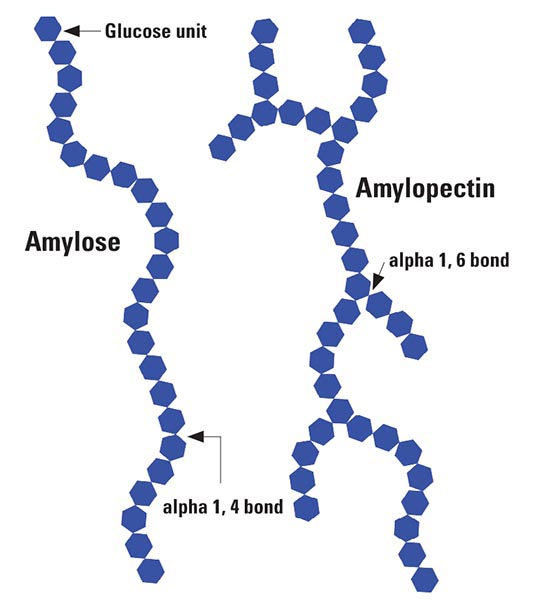
In Figure 2, it’s important to note the “branch point” is based on an α(1,6) bond. It’s not important to understand the chemical nature of that type of bond, just that it exists and that it presents some challenges. We’ll explain in a bit.
Look at all those glucose molecules. Pretty sexy, right? They are if you’re a yeast cell. Yeast makes alcohol by consuming sugar and metabolizing it. Glucose is a sugar. So is maltose, which is two glucose molecules bound together. Fructose and sucrose? Yep: sugars. We want to break those starch molecules of amylose and amylopectin chains down into simpler molecules for yeast consumption. That, my friends, is the primary purpose of mashing.
Sounds great. How do we do that, though?
Accessing the Sugars in Starch
We’ve already established that we need water, and you may have already deduced that we need heat as well. How about adding some hot water, then? That should do the trick.
If we add hot water to our milled grain (the type, consistency and process of milling is another story entirely), then that water should be able to penetrate the starch granules, opening them up to expose the starch chains. However, heat and water do not break down starch into simpler sugars — not on their own, anyway. In order to do that we need enzymes.
The important enzymes for barley are developed during malting. Contrary to popular explanations on the subject, there are actually quite a few enzymes at play during the whiskey mashing process. The ones that get talked about the most are the amylases, both alpha (α) and beta (β). Make no mistake: These are important components of our mashing system. Amylases are responsible for breaking apart much of our starch molecules into the simpler sugars we’re after.
Alpha-amylase behaves like the clumsy brute of the two. It works best at higher temperatures (optimum activity occurs between 66–71°C [150–160°F]) than β-amylase. It is also what we call a “liquefaction” enzyme because it quickly gelatinizes our starch granules. You can actually watch this process happen. Try mashing unmalted wheat or rye by itself and you’ll notice that the overall mixture is incredibly gummy and viscous. Add in 5–10% by total grist weight of malted barley and you’ll see the viscosity of your mash magically get thinner and easier to handle.
Alpha-amylase is what we call an “endo-amylase,” meaning it tends to clip starch chains at points in the middle of the chain. This is a fairly random process for α-amylase, but it goes a long way toward reducing the mash viscosity brought on by pasty starch granules. This also means that α-amylase is not really our champion when it comes to producing fermentable sugar from starch. Small amounts of sugars are produced at so random a pace that it just isn’t realistic to rely on α-amylase to give us every sugar we’re looking for.
For that we need β-amylase. Beta-amylase works at lower temperatures than its hot-headed brother, generally 54–66°C (129–150°F), with the highest activity seen around 64°C (147°F). Beta-amylase is an “exo-amylase,” which means it attacks starch chains from their ends and works its way along the chain in a methodical and linear fashion. It breaks off one molecule of maltose (a two-glucose molecule) at a time.
Amylose is easily handled by both amylases working in concert with each other. After all, amylose is essentially a straight chain of glucose molecules, so α-amylase and β-amylase have no issues with converting it to simpler sugars.
Amylopectin, with its myriad of branch points, is another story entirely. Beta-amylase moves along the amylopectin chains until it gets within two or three glucose units of an α(1,6) branch point before it has to abandon ship and move on to another less-encumbered starch chain. But remember, these branch points are also composed of glucose molecules, so we don’t want to leave them alone.
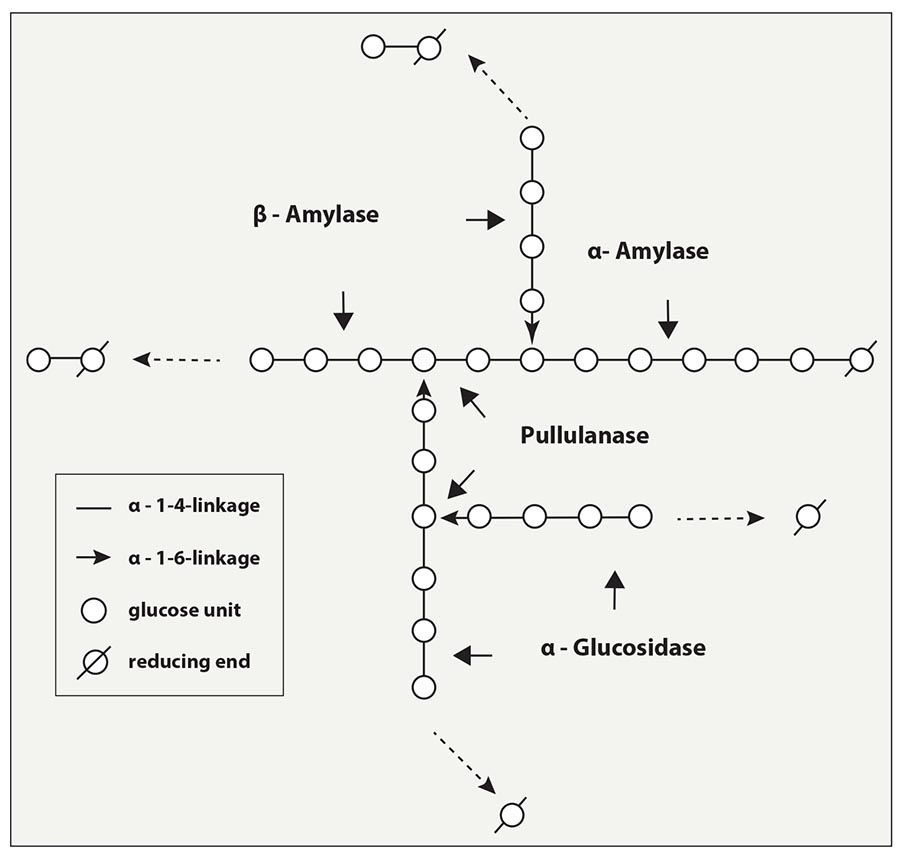
So how do we break down the branch points and increase our overall wort fermentability? This is where our secondary enzymes come into play. Limit dextrinase (sometimes referred to as pullulanase) has the ability to break apart α(1,6) bonds. Amyloglucosidase breaks down larger sugars like maltotetrose (a four-glucose molecule) and maltotriose (a three-glucose molecule) down into singular glucose molecules (see Fig. 3).
But here’s the catch with these secondary enzymes: Compared to their amylase cousins, they aren’t very active during normal mashing conditions. However, they do become more active during fermentation and give us what we call a “secondary conversion.” This secondary conversion actually increases the amount of fermentable sugars by upward of 15%, which is a huge boost to our plant efficiency. Brewers boil their wort after mashing to sanitize it and for hop utilization (among other things), which denatures all the mash enzymes, especially our secondary enzymes. Since distillers typically don’t boil their wort, we get to keep these valuable enzymes in play for more starch conversion to take place (see Fig. 3).
What You Need to Know About Water
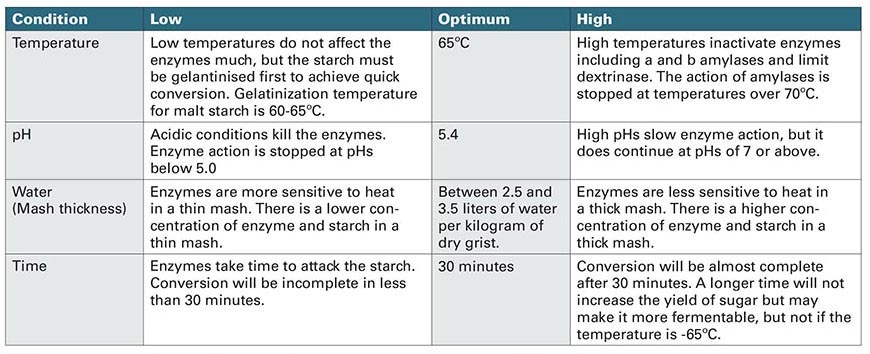
We’ve talked about starch and the enzymes to break it down into fermentable extract, but we’ve only danced around the factors that make those enzymes active. Clearly, we need water, as it is required to penetrate the starch granules. Heat added to the water aids in gelatinizing the starch and as we mentioned a moment ago, our amylases have preferred temperature ranges. So, what’s the correct mash temperature for grain spirits production?
The answer (sort of) depends. First off, what we’re talking about here is “saccharification” temperature, which is where our amylases do their jobs. In that case we want to think about what each amylase is doing. Generally, for distilling purposes, we’re interested in obtaining the most fermentable wort possible. For that, β-amylase is our guy and has an optimum activity at 64°C (147°F). Notice that this falls just shy of our preferred temperature range for α-amylase activity, but that’s okay here. Alpha-amylase still works suitably well at 64°C, breaking apart starch chains and making them more easily attackable by β-amylase.
Next to the temperature of the water, we have to talk about the amount of water. This is essentially a question of mash thickness. There are pros and cons for thin mashes (lots of water) to thick mashes (less water). Thin mashes are much easier to work with than thicker mashes, as they have more water to aid in the movement of grain solids from place to place. The drawback is that with thin mashes you have to be much more careful with monitoring your mash temperature. Thin mashes don’t provide much in the way of insulation for enzymes, and small changes in mash temperature can have dramatic effects on enzyme performance. These concepts work the opposite way for thick mashes: more enzyme heat insulation but harder to work with. So, what’s the sweet spot? It depends on who you talk to since every system, distiller and recipe is a little bit different. However, 1.5 quarts of water per pound of grain works well for most folks. (In metric, you’re looking at roughly 3 liters of water per kilogram of grain.)
The other key parameter at play that we haven’t discussed is mash pH. The pH of a system is simply a way to measure how acidic or basic the system is. Mash pH is incredibly important for enzyme activity. Without getting into the chemistry too much, pH affects the shape of the enzymes that we care about. If the pH isn’t within a suitable range, our amylases and secondary enzymes won’t function well and, in some cases, not at all. The accepted pH range for mashing is 5.2–5.8, with 5.4 considered the optimum. Above this range and starch conversion will still occur but at a significantly slower pace. Below 5.0, the amylase enzymes stop working altogether.
Finally, we need to discuss how long the mash should take. Once again, this depends on what you’re talking about when it comes to “mashing,” which for the moment is simply the saccharification step. The amount of time needed for conversion is affected by several factors that get too complicated to discuss in detail here. Things like mash thickness, temperature, pH, recipe composition and malt modification all play a role. However, let’s keep things simple. In general, a saccharification rest should last anywhere from 30 minutes for high amounts of malted grains in the recipe to 60 minutes for low amounts of malted grains. See Table 1, page 126 for a handy summary of all these factors.
Getting Sugar from Other Grains
Up to this point we’ve really only talked about mashes with high amounts of barley in them, which excluding the burgeoning American single malt whiskey category is not that common in the United States. What if we’re making a high-corn bourbon or rye whiskey? How does the mash chemistry work in those systems? These types of whiskeys have large amounts of unmalted or “adjunct” grains in them, which does change the processing steps a little bit.
Fortunately for us, the primary objectives for mashing unmalted grains are pretty much the same. We have a source of starch that we need to convert into simpler sugars. Unfortunately, because the grain isn’t malted, our grains don’t have much in the way of saccharification enzymes to help us along. Also, remember what we talked about with starch granules earlier: Not all starch behaves the same way.
First off, different starches require different temperatures to properly gelatinize. Conveniently for brewers using barley, the gelatinization temperature for barley starch is in a similar range to the amylase enzyme activity optimum. However, for corn starch the gelatinization temperature is significantly higher. Corn starch granules are typically small in size (Type B), polyhedral in shape and have low amylopectin contents (see Fig. 4). This makes water penetration into the granule difficult at lower temperatures. Therefore, it is common to see recommended corn starch gelatinization temps of 70–80°C (158–176°F). In practice, however, most corn whiskey distillers will tell you that unless you want to wait around all day for your corn to gelatinize, you’re better off kicking that temperature up to 90–100°C (194–212°F). For a list of starch gelatinization temperatures see Table 2, page 127.
This “cooking” of the grain is obviously energy-intensive. It’s therefore not uncommon for distillers to add commercially prepared exogenous enzymes to their mashes to help push things along. In the case of corn, there are several high-temperature amylase preparations (typically fungus-derived) that can be dosed into the cooker. Depending on the product this can lead to several benefits. First is the quick reduction of viscosity in the cooker. Second is gelatinizing of starch at lower temperatures, reducing energy needs. However, many bourbon distilleries operate just fine without the addition of enzymes, so it comes down to a matter of preference. Regardless, the cooking of corn generally lasts anywhere from 30–60 minutes depending on the distiller and the specific process they’re using. If you’re cooking at a higher temperature, then less time is necessary to achieve the desired results.
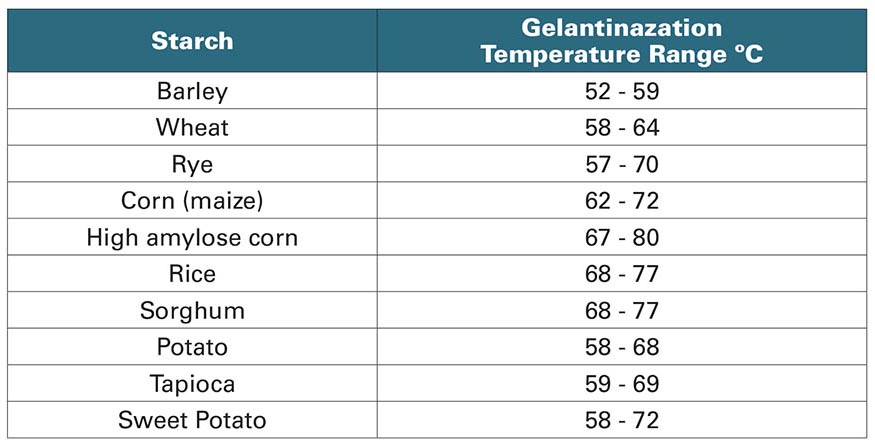
Note in Table 2 that rye starch has a higher gelatinization temperature range than barley does. There is some overlap there, but rye skews closer to the 70°C (158°F) mark than barley does. At that temp we don’t get much in the way of β-amylase activity, so many distillers opt to cook their corn at 70°C (158°F). You might be wondering why they wouldn’t just cook it at 90°C (194°F) or higher if they’re doing, say, a bourbon with rye. That’s a valid point, but there’s a problem there. First, adding more grain requires more heat to gelatinize all the starch in the system. Secondly, at least anecdotally, many distillers report that cooking rye at too hot a temperature causes a lot of the natural “gummy” compounds in rye (beta-glucans and arabinoxylans, mostly) to cause problems with mash viscosity. Research on rye viscosity is somewhat scant but what little has been done, does suggest that extraction of arabinoxylans increase with temperature (https://link.springer.com/article/10.1186/s40538-017-0096-6#citeas). Arabinoxylan contents can be reduced somewhat by using malted rye that was put through a long and cool germination period. However, if you’re using malted rye, then you’ve paid for the enzymes and you wouldn’t want to denature them by cooking above 70° C (158° F).
This is why during a mash with unmalted (adjunct) grains, there is generally a step-down cooking process. We start at 90°C (194°F) for corn and sit there for 30 minutes or so. Then we cool down to 70°C (158°F) and add our rye and sit for another 30 minutes before finally cooling to our saccharification temperature of 64°C (147°F).
There’s a lot more to cover with mash chemistry, but we’ve tried to cover the basics here. Hopefully this gives you a better understanding of what’s happening in your mash whether you’re a bourbon maker or single-malt guru. Better whiskey starts with your materials and your mash technique. Happy brewing!