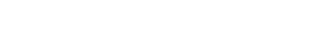As a rum distiller, I’m always on the lookout for novel production methods capable of increasing the organoleptic complexities of pot-distilled rum. Often you hear enthusiasts wax poetically about Jamaica’s famed dunder pits and their historic use, other fermentation additives (muck and cane vinegar), or even the legendary Cousins’ Process for production of high ester rums. Clearly, there are many ways to produce organoleptically complex rums, either adapting the Jamaican methods to your production process or through raw material selection/sourcing, yeast(s) selection, and fermentation and distillation method(s). Recently, industrial suppliers like Lallemand and Fermentis have released commercial microbial tools that we can add to our distilling toolbox. Prepare yourself, reader, for I speak of…bacteria!
For those of us who practice the fermentative arts, the idea of purposefully introducing bacteria into our fermentations may cause some mild terror; however, incredible things await us when we work together with these microbes instead of fearing them. Over the summer of 2022, as part of an ADI Distilling Research Grant and my Heriot-Watt MSc thesis research, I had a chance to study how the careful administration of bacteria to a rum fermentation can produce new compounds for yeast to metabolize and thus yield new aromas and flavors in one’s spirit. Long story short: You have to be very careful when working with bacteria, but it’s not as scary as it sounds.
The Project
This study investigated the effects of co-inoculation of a yeast (Lalvin EC-1118™) and three commercially available lactic acid bacteria (DistilaBact® LP, EnoFerm Alpha™, SafSour LP 652™) on the organoleptic properties of un-aged, pot-distilled rum produced from Louisiana blackstrap molasses and raw cane sugar. The bacteria were expected to increase the presence of specific acids during fermentation, which the yeast would metabolize into esters or ester precursors.
All trials were performed at Windon Distilling Company, the home of LYON RUM, in St. Michaels, Maryland. On average, the distillery produces 80 gallons of still-strength rum per week and, like many small producers, does not have modern laboratory equipment that allows in-depth analysis of fermentations (i.e., plating and culturing, cell counts, microscopy, etc.) and distillates (i.e., GC-MS and others). If this sounds a lot like your current production facility, good news! You, too, can begin experimenting with different bacteria strains to improve the aroma and flavor characteristics of your products!
Materials
The blackstrap molasses and raw cane sugar used are both non-GMO products of the Lula-Westfield Sugar Factory of Paincourtville, Louisiana.
- Lalvin EC-1118™: a commonly chosen yeast and true industry workhorse, noted for its fermentation performance, neutral organoleptic profile, and its ability to showcase the raw ingredients of a mash bill
- DistilaBact® LP: a lactic acid bacteria product capable of producing sour mash-related organoleptic properties in distilled spirits, such as lactic (creamy) and citrus and tropical fruit notes
- EnoFerm Alpha™: a malolactic fermentation bacteria product typically used in the wine industry to add roundness, mouthfeel, red fruit, pear, and tropical fruit notes to wines
- SafSour LP 652™: a kettle-souring bacteria used in the brewing industry to add citrus, tropical, and other fruity notes to a variety of beer styles
Methods
All research trials were performed in triplicate. All vessels and equipment were cleaned and sanitized before use. The yeast and bacteria were both rehydrated and added to the fermentation according to manufacturer directions. Fermentation performance was tracked using a standard glass fermentation hydrometer. The pH meter was calibrated weekly using pH 7 and pH 4 solutions, and the measuring tip was stored in an Oakton storage solution when not in use. For stripping runs, a standard glass alcohol hydrometer was used to track starting and ending ABV.
The following measurements were taken daily:
- Specific gravity
- pH
- Temperature
- Visual/sensory check of activity
Fermentation was complete when there was no change in specific gravity within a 24-hour period and no fermentation activity was visually present. The experimental setup can be seen in Figure 1, with each trial having an A, B, and C segment.
Fermentation and Distillation
All fermentations were 6 to 7 days in length and 20 gallons in volume, with a composition of 24 lbs of blackstrap molasses, 20 lbs of raw cane sugar, and 138.4 lbs of filtered municipal water. Fermentations took place in lidded 55-gallon stainless steel drums. The water was heated to 88–93°F before being added to the drum for all but the EnoFerm Alpha™ trial. There, the water was heated to 84–85°F to ensure that the pitch temperature was below 86°F and that the fermentation temperature would drop below 81°F when the ABV was about 10%. Fermentation temperatures were not controlled.
The first distillation was performed in a 26-gallon stainless steel pot still heated with an internal electric element. Once heated, the still operated at 11 amps (out of 20) for an average length of 8.4 hours. Low wines were collected into five-gallon glass carboys, with initial and final ABVs of 66% and 16%, respectively, and a total of four gallons of low wines at 42% ABV per run.
The second distillation was performed in a three-gallon copper alembic pot still heated by an electric hot plate. Once heated, the hot plate operated at a heat setting of 4.5 (out of 5). The first set of spirit runs were distilled entirely from three gallons of low wines and will be referred to as control, trial 1, trial 2, and trial 3. For each trial triplicate, the heads, hearts, tails, and remaining low wines were blended to create respective aggregates.
A subsequent production-style spirit run was then performed using a blend of low wines (85%), heads (10%), and tails (5%) and will be referred to as control (WDC), trial 1 (WDC 1), trial 2 (WDC 2), and trial 3 (WDC 3). All hearts were then slowly proofed down to 45% ABV using carbon-filtered municipal water. The choice of 45% ABV was not arbitrary and served as a point of comparison to our standard white rum, which is bottled at 45% ABV.
Data Analysis
For each trial, samples were taken from the proofed aggregate hearts and brought to Brewing and Distilling Analytical Services (BDAS Testing, https://bdastesting.com) in Lexington, Kentucky, for gas chromatography and sensory panel analysis. After testing, the data was analyzed using Microsoft Excel.
Fermentation Results
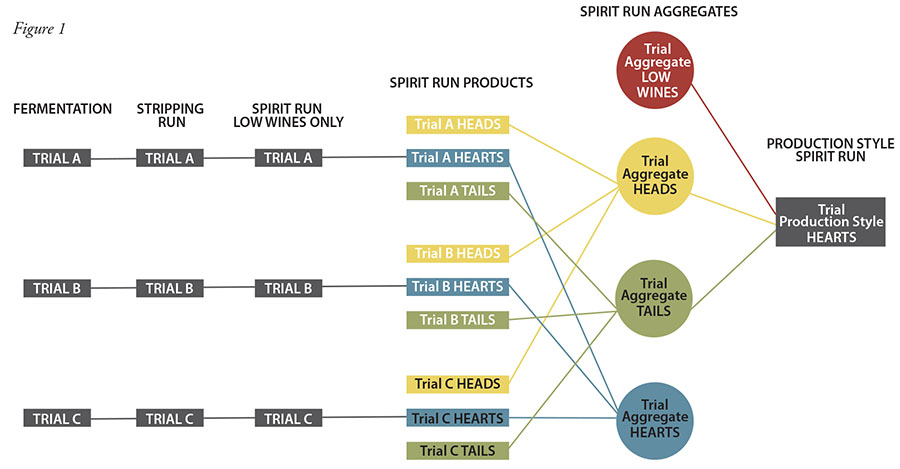
Fermentation performance was largely unaffected, with little difference between final levels of trial pH, specific gravity, and ABV, versus the control, except trial 2, which had a significantly higher pH. The results can be seen in Figure 2.
It was surprising to see how EnoFerm Alpha™ affected the fermentation pH. Typically, when bacteria are involved, or worse, when they are directly competing with the yeast, the pH rapidly drops to a point where the yeast can’t do their job, usually in the pH 2–3 range. This also tends to correspond with a stalled drop in specific gravity. Here, though, the pH drop stalled just under pH 5.0. Upon sensory inspection, the fermentation did smell slightly different than the others, with a very faint phenolic characteristic that later presented in the sensory panel analysis.
GC Results
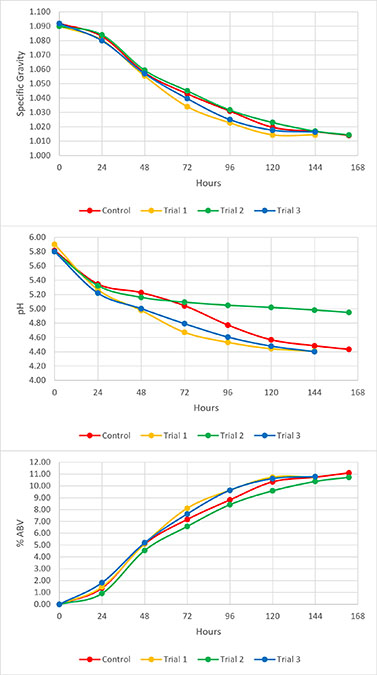
Gas chromatography showed that all trials had similar concentrations of higher alcohols and esters to their respective controls, except for isobutanol and active and iso-amyl alcohol, which were distinctly different for trial 1, trial 2, WDC 1, and WDC 2. Additionally, the total fusel oil content, which is the sum of n-Propanol, isobutanol, 1-Butanol, and active and iso-amyl alcohols, was distinctly different in trials 1–3, WDC 1, and WDC 2. The results can be seen in Figure 3.
Sensory Panel Results
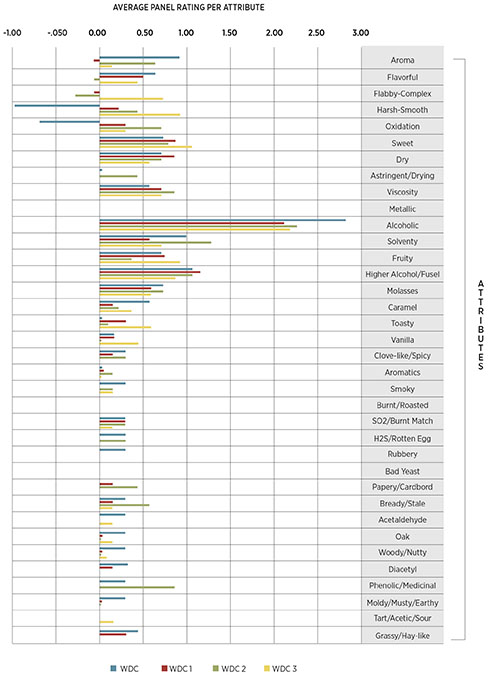
A trained sensory panel evaluated all distillates using a 36-point attribute ballot, including low wines and production-style distillations. Each trial was found to be distinctly different than its respective control, with trial 2 having a greater overall score than the control (1.11 vs. 0.43) and WDC 3 scoring higher than WDC (0.54 vs. 0.40). The overall results for the finished (two-pass) distillations can be seen in Figure 4. Note that negative numbers represent negative answers for binary choice questions.
We also evaluated each distillate set and found them to be distinctly different than their respective controls, with trial 1 preferable to the control and WDC 1 preferable to WDC. Those qualitative results can be seen in Figure 4.
Discussion
This project demonstrated that co-inoculating yeast and bacteria can increase the organoleptic characteristics of pot-distilled rum with minimal additional process complexities. That’s a science-y way of saying that those tropical/citrus notes and enhanced creaminess mentioned in Figures 4 and 5 lead to many future product ideas.
Imagine aging this rum for 2-10 years in an ex-bourbon, Cognac, or Calvados barrel and think about what a layered and flavorful experience it would be. Honey, butterscotch, caramel, vanilla, hints of baking spices, apple, and oak building off that base of tropical/citrus notes and creaminess, leading to a truly remarkable in-glass experience. Delicious! It would also be stellar as an aged or unaged liqueur base and could play well with cream, coffee/chocolate, vanilla, citrus peels, herbs, and tropical spices. Alternatively, using a different yeast strain in concert with these bacterial tools would further open the doors of possibility. Truly exciting times to be a distiller!
Pointers on Working with Bacteria
I hope this study got you excited about ways to experiment in your distillery. How to actually bring bacteria into your distillery deserves its own article, but rum fermentation research isn’t only the purview of larger producers with state-of-the-art labs. It can be easily performed by craft and micro-distilleries.
There are, however, a few best practices that can get you safely started:
- Depending on the sensitivities of your fermentations, it is best practice to keep the bacteria isolated from your main fermentation area, particularly if your fermenters are open to the atmosphere.
- To ensure the best chance of success, follow all manufacturer recommended best practices for using your chosen bacteria strain(s), including rehydration temperatures and times, the timeline for adding it to your fermentation, and the fermentation temperature and pH ranges.
- Thoroughly sanitize and sterilize all equipment, tanks, and vessels used for any part of your bacteria program, particularly if these items are shared across your facility. This will significantly reduce the chance of cross-contamination before and after use.
Beyond the organoleptic front, I hope that this research kick-starts a larger conversation between distillers and yeast and bacteria suppliers to continue pushing the boundaries on usable yeast and bacteria and lead to an open-source, free exchange of ideas which benefits the entire industry. In the not too distant future, I can see these suppliers offering “starters” which are combinations of yeast and bacteria, tailored to provide specific profiles based on base raw ingredients.