Introduction
Cinchona is a genus of evergreen tree in the Rubiaceae (coffee) family, native to the Andean forests of South America and naturalized north into parts of Mesoamerica. However, most of the global supply is cultivated in Southeast Asia.
History
In the Andes, cinchona bark has long been used by the indigenous Quechua peoples for its antipyretic (fever-reducing) and muscle relaxant properties. In the colonial period, it was widely used to treat malaria, most famously by Robert Talbor to cure King Charles II of England and then the son of King Louis XIV of France in 1679–1680. Cinchona extracts and purified alkaloids thereof (predominantly quinine, but not exclusively) remained the only effective antimalarial treatment until the early twentieth century. Quinine was first isolated in 1820. Quinine is still used to treat drug-resistant malaria, as well as the tick-borne babesiosis, and is on the World Health Organization’s List of Essential Medicines.
Historically, wine or spirit was typically used for extraction. Cinchona bark is fairly unpalatable on its own, so other ingredients were often added to balance the flavor and/or confer additional properties unto the concoction. Sweeteners could also be added. It is used as a bittering agent in beverages such as amaro, Barolo Chinato and some tonic water.
Flavor & Extraction
Cinchona bark contains the bitter alkaloids quinine, quinidine, cinchonidine and cinchonine, as well as the bitter glycoside, quinovin. Cinchona bark is also high in easily-oxidized tannins (primarily cinchotannic acid), giving the bark an astringent quality as well as its reddish color. None of these compounds are volatile, so will not be extracted by conventional distillation, and the essential oil content of the bark is low and heavy. They are also not very soluble in water, so efficient extraction requires a solvent such as ethanol or glycerin. If ethanol is used as a solvent, is can be easily evaporated off through the application of heat, such as when reducing the solution down to a syrup. If the bark is simply boiled in water, much less will be extracted, but fine particulate matter in suspension (i.e., powder) can have a high alkaloid content.
Most modern tonics do not use the bark itself or a full spectrum extract, but rather purified quinine. In order to incorporate the compound into a water-based beverage, either quinine hydrochloride or quinine sulfate salts are typically used.
Alkaloid Content
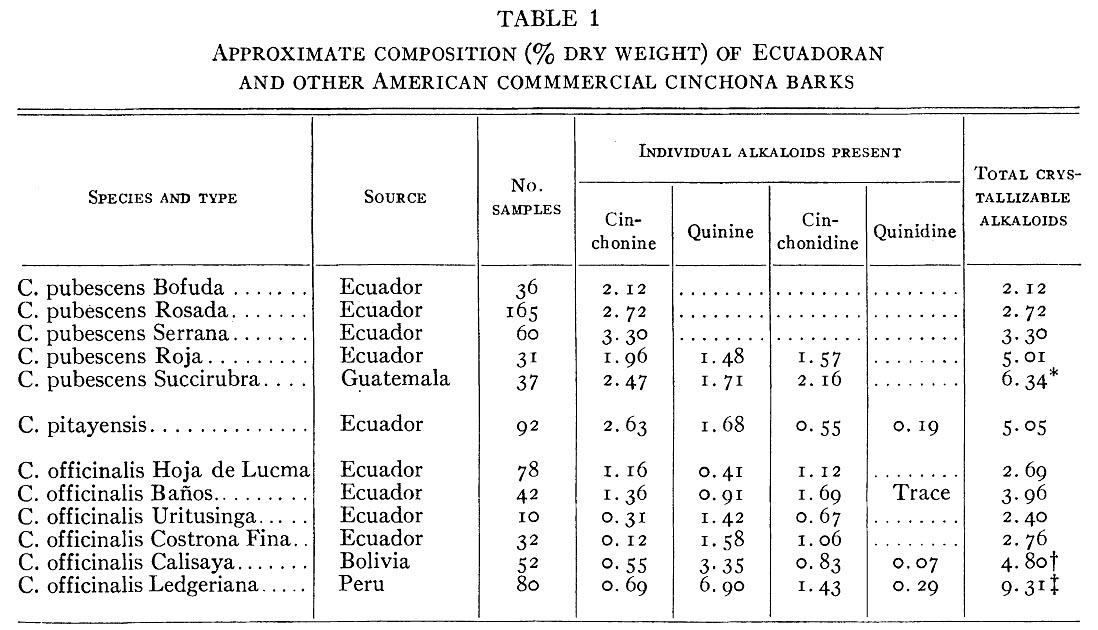
There are 25 known species within the genus Cinchona but only a few are widely cultivated. Cinchona bark has a wide range of total and specific alkaloid content, influenced not only by species but also growing conditions. Quinine is usually the dominant alkaloid (typically 50–90% of the total), but some wild species contain almost none. Bark from South America tends to have lower quinine and total alkaloid content than that from the plantations of Southeast Asia.
This is because the latter are primarily used for industrial alkaloid extraction, while the former are more commonly used in a less processed format. Total alkaloid content of cinchona bark can range from 2 to 17% by mass.
Limit & Toxicity
Cinchona bark and quinine are approved by the FDA as “food additives,” but there is an upper limit of 83 ppm (mg/l) total cinchona alkaloids in the finished beverage. Similarly, the EU limit is 100 ppm. For comparison, the recommended daily quinine dose to treat malaria is 648 mg — equivalent to 7.8 liters of tonic at maximum legal quinine concentration!
While the cinchona alkaloids do have therapeutic value, they are also toxic at high doses. The most common side effect of a toxic dose of quinine is cinchonism. Symptoms can include tinnitus, hearing impairment, headache, nausea, vomiting, diarrhea, visual disturbances and postural hypotension. Extremely high doses of quinine or quinidine can be fatal.
Therefore, it is crucial to understand these limits when using cinchona bark. When in doubt regarding the amount of alkaloids extracted, err on the side of caution. If a product contains significant particulate matter, ensure that it is well mixed immediately prior to each use to avoid uneven distribution of alkaloids.
COVID-19, Chloroquine & Hydroxycholoroquine
In 1934, Hans Andersag synthesized chloroquine based on the structure of quinine. Hydroxychloroquine (HCQ) was synthesized by both sides during World War II based on chloroquine. Like quine, both of these drugs are on the WHO’s List of Essential Medicines as antimalarials (AMs) and are also used to treat inflammatory autoimmune diseases such as lupus and rheumatoid arthritis. They also have the potential for serious side effects. Additionally, because they are similar in structure and bioactivity, their side effects can be compounded by the consumption of quinine. Despite the aforementioned similarities, these drugs have substantial structural differences from quinine and cannot be used interchangeably.
There has been much discussion of the use of chloroquine and hydroxychloroquine to treat the COVID-19 disease caused by the Sars-CoV-2 virus, not all of it grounded in fact. The current body of scientific research does not support this use and HCQ has been associated with cardiac toxicity in COVID-19 patients. Even if these drugs are effective against COVID-19, that does not mean that quinine or other cinchona alkaloids will be as well. And, as previously noted, you would need to drink inhuman amounts of tonic water to get a therapeutic dose of quinine.
References
Argumánez, C. M., Fuente, I. S., Collado, Z. M., Pita, D. S., Gómez, B. M., & Izquierdo, J. S. (2017). Hydroxychloroquine, a potentially lethal drug. Medicina Intensiva, 41(4), 257-259. doi:10.1016/j.medine.2016.05.003
Eyal, S. (2018). The Fever Tree: from Malaria to Neurological Diseases. Toxins, 10(12), E491. doi:10.3390/toxins10120491
Hoffmann, C. (2020, April 27). Covid-19: FDA Issues Hydroxychloroquine Warning. Physician’s Weekly.
Kacprzak, K. (2013). Chemistry and Biology of Cinchona Alkaloids. In R. K., & M. J. M. (Eds.), Natural Products. Berlin, Heidelberg: Springer. doi:10.1007/978-3-642-22144-6_22
Klein, W., & Pieters, T. (2016). The Hidden History of a Famous Drug: Tracing the Medical and Public Acculturation of Peruvian Bark in Early Modern Western Europe (c. 1650-1720). Journal of the History of Medicine and Allied Sciences, 71(4), 400-421. doi:10.1093/jhmas/jrw004
Martin, W. E., & Gandara, J. A. (1945). Alkaloid Content of Ecuadoran and Other American Cinchona Barks. International Journal of Plant Sciences, 107(2), 184-199.
Vinetz, J. M. (2020). Lack of efficacy of hydroxychloroquine in covid-19. BMJ, 369. doi:10.1136/bmj.m2018
Walker, K., & Nesbitt, M. (2020). Just the Tonic: A Natural History of Tonic Water. Kew: Royal Botanic Gardens.